|
# # # # Alpha synuclein is one of the most common proteins in our brains and it has long been associated with Parkinson’s. The protein appears to clump together forming dense clusters ( or “aggregates“) in the Parkinsonian brain, and this may be related to the progressive neurodegeneration. Researchers have been desperately seeking small molecules that will break up (or dissociate) these aggregates in the hope that it will slow down the progression of PD and allow neurons to return to health. One example of such a molecule is UCB0599, which is being clinically developed by the pharmaceutical company UCB. This week, UCB presented the first clinical results for UCB0599 from their Phase I trial. In today’s post, we will look at what alpha synuclein is, review what is known about UCB0599, discuss the results of the study, and consider what comes next. # # # # |
 Source: AAN
Source: AAN
Last week at the 2021 American Academy of Neurology virtual meeting a poster was presented by the pharmaceutical company UCB.
Here at SoPD HQ, we have been eagerly awaiting these results.
They were the findings from the first Phase I clinical trial of a new molecule called UCB0599.
What is UCB0599?
UCB0599 is a brain-penetrant, oral small molecule alpha-synuclein misfolding inhibitor.
What does that mean?
Alpha synuclein is one of the most abundant proteins in our brains – making up about 1% of all the proteins floating around in each neuron in your head – and it is a very well studied protein (with over 13,000 research reports listed on the Pubmed search engine with the key words ‘alpha synuclein’). The attention focused on alpha synuclein is partly due to its association with Parkinson’s.
How is alpha synuclein associated with Parkinson’s?
In 1997, this research report was published:

Title: Mutation in the alpha-synuclein gene identified in families with Parkinson’s disease.
Authors: Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A, Pike B, Root H, Rubenstein J, Boyer R, Stenroos ES, Chandrasekharappa S, Athanassiadou A, Papapetropoulos T, Johnson WG, Lazzarini AM, Duvoisin RC, Di Iorio G, Golbe LI, Nussbaum RL.
Journal: Science. 1997 Jun 27;276(5321):2045-7.
PMID: 9197268
In this study, the researchers described multiple families of Italian and Greek origins that had numerous members affected by Parkinson’s. All of the afflicted individuals carried tiny genetic errors (or variations) in the region of DNA that was responsible for providing the template (or RNA) that is used to produce alpha synuclein protein.
With this and subsequent research findings, alpha synuclein became the first genetic risk factor associated with Parkinson’s.
And the researcher community were quick to start exploring the biology of this protein within the context of Parkinson’s.
In fact, just two months after the publication of the report above, the results of another study were published that would further cemented alpha synucleins place in the world of Parkinson’s:

Title: Alpha-synuclein in Lewy bodies.
Authors: Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M.
Journal: Nature. 1997 Aug 28;388(6645):839-40.
PMID: 9278044
In this study, the investigators showed that alpha synuclein was present in Lewy bodies – one of the characteristic features of the Parkinsonian brain:

Alpha synuclein (brown staining) in Lewy bodies. Source: Nature
For those interested in the history of alpha synuclein research on Parkinson’s, click here to read a previous SoPD post devoted to this topic.
What are Lewy bodies?
Lewy bodies are dense circular clusters of aggregated alpha synuclein protein (and other proteins and lipids) that are found in specific regions of the brain in people with Parkinson’s (Click here for more on Lewy bodies).
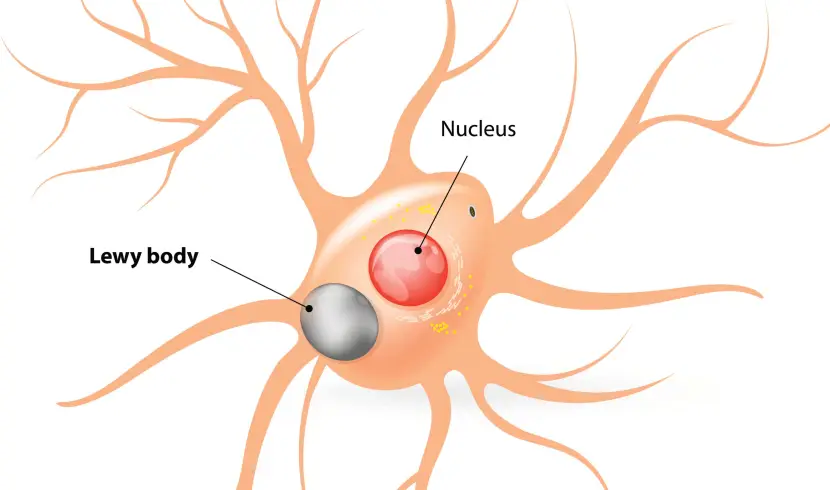 A cartoon of a neuron, with the Lewy body indicated within the cell body. Source: Alzheimer’s news
A cartoon of a neuron, with the Lewy body indicated within the cell body. Source: Alzheimer’s news
Lewy bodies are referred to as a cellular inclusion, as they are almost always found inside the cell body:
 A photo of a Lewy body inside of a neuron. Source: Neuropathology-web
A photo of a Lewy body inside of a neuron. Source: Neuropathology-web
But it should be noted that aggregated alpha synuclein protein is not limited to just the Lewy bodies. In the image below, alpha synuclein has been stained brown on a section of brain from a person with Parkinson’s. In the affected areas of the Parkinsonian brain, aggregated alpha synuclein can be seen in the branches (or neurites; see black arrow in the image below) of cells.

Examples of Lewy neurites (stained in brown; indicated by arrows) from a human brain. Source: Wikimedia
Why does alpha synuclein aggregate in the Parkinson’s brain?
It’s a really good question. Holy grail kind of stuff. And the short answer is: We don’t really know.
What we do know it that when alpha synuclein protein is produced by a cell, it normally referred as an ‘unfolded protein’, in that it is does not really have a defined structure. It is considered intrinsically disordered.
In it’s original form, alpha synuclein is considered a monomer – which is a single molecule, just one copy of the protein. It is capable of binding to other molecules, and when it binds to other alpha synuclein proteins, they form what is called an oligomer (a collection of monomers). And these oligomers can have different structures.
In Parkinson’s, alpha synuclein can also bind (or aggregate) with other alpha synuclein proteins to form what are called ‘fibrils’.

Microscopic images of monomers, oligomers and fibrils. Source: Brain
And it is believed that these fibril forms of alpha synuclein protein may go on to help produce the Lewy bodies that characterise the Parkinsonian brain.
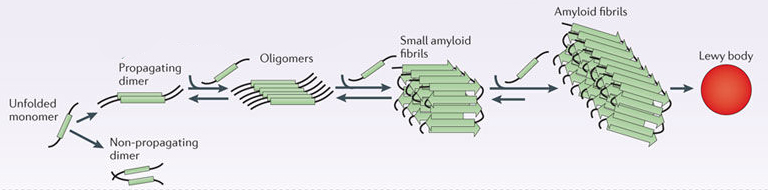 Parkinson’s associated alpha synuclein. Source: Nature
Parkinson’s associated alpha synuclein. Source: Nature
This aggregated form of alpha synuclein may also be toxic at high levels, which may be causing the loss of certain neurons in the brains of people affected by Parkinson’s. There are also theories being tested that alpha synuclein may be involved in the progression of the condition.
But if alpha synuclein protein is inside of cells, how would it be involved with the progression of Parkinson’s?
So back in the 1990s, there were a series of clinical trials of cell transplantation conducted on people with Parkinson’s. The idea was to replace the cells that have been lost to the condition (Click here to read a previous post about cell transplantation). Many of the individuals who were transplanted have now passed away by natural causes and their brains have been examined post-mortem.
One very interesting finding from the analysis of those brains is that some of the cells in the transplants have Lewy bodies in them (up to 10% of transplanted cells in one case – Click here to read the research report on that case).
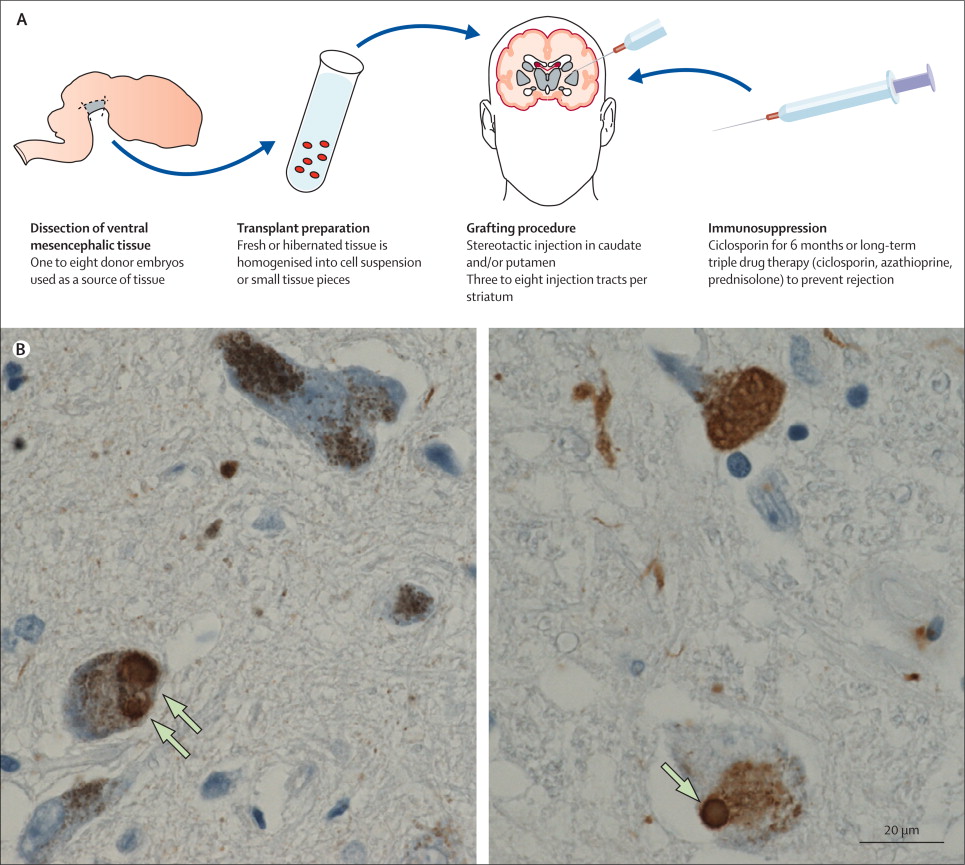 Above are photos of neurons from the post-mortem brains of people with Parkinson’s that received transplants. White arrows in the images above indicate lewy bodies inside transplanted cells. Source: The Lancet
Above are photos of neurons from the post-mortem brains of people with Parkinson’s that received transplants. White arrows in the images above indicate lewy bodies inside transplanted cells. Source: The Lancet
This finding suggested to researchers that somehow this neurodegenerative condition is being passed on from the Parkinson’s affected brain to the healthy transplanted cells, via cell-to-cell transfer.
And researchers have proposed that the aggregated form of alpha synuclein may be the guilty party in this cell-to-cell transfer process – being passed from one cell to another and seeding further protein aggregation as it gets passed along (Click here to read more on this idea and the experimental evidence for it).
So aggregated alpha synuclein may be toxic, help to produce Lewy bodies, and be involved with the progression of Parkinson’s?
That’s the theory.
And this is the reason why researchers and biotech companies have been working very hard to develop molecules like UCB0599 – a brain-penetrant alpha-synuclein misfolding inhibitor. To stop the production of the aggregated form of alpha synuclein.
|
# RECAP #1: Alpha synuclein is one of the prime suspects in the hunt for an influential agent in the progression of Parkinson’s. This protein clusters together in the Parkinson’s brain, possibly helping to form Lewy bodies and it may be aiding in the progression of the condition. Research groups around the world have been focused on identifying molecules that can stop or reduce this aggregation in an effort to slow down the progression of the condition. # |
So what do we know about UCB0599?
UCB0599 was originally discovered by a biotech company called Neuropore Therapies.
The company was looking for molecules that reduce alpha synuclein toxicity by displacing alpha synuclein from the membrane.
Why does that mean?
Alpha synuclein is known to bind to the inside wall of cells (or their membrane). As we discussed above, floating around by itself, the shape of alpha synuclein protein is intrinsically disordered (see panel A below), but once it interacts with the membrane it adopts a specific structure (called an alpha helical structure – see panel B below). This process allows the protein to facilitate activity involved with cell-to-cell communication (synaptic vesicle fusion and release).
 Source: Semanticscholar
Source: Semanticscholar
At high concentrations, however, membrane-bound alpha synuclein can disrupt lipid membranes, which has been suggested as a possible mechanism of toxicity. In addition, membrane-bound alpha synuclein can adopt a structure that encourages oligomers to form, which may lead to the aggregated form of alpha synuclein we discussed above.
The researchers at Neuropore Therapies used molecular modelling techniques to design a molecule for the task of displacing alpha synuclein from the membrane. They called this molecule NPT100-18A.
In 2016, they published this report describing the development and characterisation of NPT100-18A:
 Title: A de novo compound targeting α-synuclein improves deficits in models of Parkinson’s disease.
Title: A de novo compound targeting α-synuclein improves deficits in models of Parkinson’s disease.
Authors: Wrasidlo W, Tsigelny IF, Price DL, Dutta G, Rockenstein E, Schwarz TC, Ledolter K, Bonhaus D, Paulino A, Eleuteri S, Skjevik ÅA, Kouznetsova VL, Spencer B, Desplats P, Gonzalez-Ruelas T, Trejo-Morales M, Overk CR, Winter S, Zhu C, Chesselet MF, Meier D, Moessler H, Konrat R, Masliah E.
Journal: Brain. 2016 Dec;139(Pt 12):3217-3236.
PMID: 27679481 (This report is OPEN ACCESS if you would like to read it)
The researchers found that in the presence of NPT100-18A (red pentagon in the schematic below) the conformation of alpha synuclein changed, resulting in its release from the membrane (the grey collection of circle in the schematic below).
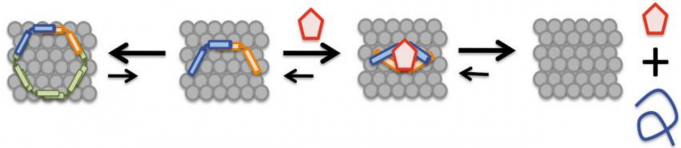 Source: PMC
Source: PMC
And they found that NPT100-18A improved motor problems, decreased the accumulation of alpha synuclein, and limited the neurodegeneration that is seen in the brains of mulitple genetic mouse model of Parkinson’s (the mThy1-α-syn WT and mThy1-α-syn E57K models).
This proof-of-principle result was very encouraging for the Neuropore Therapies investigators who began developing a next generation version of the molecule.
Why? Why didn’t they just take NPT100-18A into clinical trials?
NPT100-18A had limited oral bioavailability (meaning it didn’t get into and last long in the body) and relatively poor brain penetration (<11% of plasma levels), so it would have been difficult to take into clinical trial. The next generation molecule that Neuropore Therapies developed, however, was much better.
It was called NPT200-11.
The development and characterisation of NPT200-11 is described in this publication:
 Title: The small molecule alpha-synuclein misfolding inhibitor, NPT200-11, produces multiple benefits in an animal model of Parkinson’s disease.
Title: The small molecule alpha-synuclein misfolding inhibitor, NPT200-11, produces multiple benefits in an animal model of Parkinson’s disease.
Authors: Price DL, Koike MA, Khan A, Wrasidlo W, Rockenstein E, Masliah E, Bonhaus D.
Journal: Sci Rep. 2018 Nov 1;8(1):16165.
PMID: 30385782 (This report is OPEN ACCESS if you would like to read it)
The results of this study demonstrated that NPT200-11 was a safe molecule with more properties suitable for clinical evaluation (than NPT100-18A). NPT200-11 also reduced alpha synuclein pathology in a genetic mouse model of Parkinson’s (the Line 61 mouse model overexpressing human wild type alpha synuclein).
Further analysis also demonstrated that NPT200-11 reduced neuroinflammation, normalized levels of the dopamine-related proteins (such as DAT) in the brain, and improved motor function in the mice.
So what happened next?
In January 2015, Neuropore Therapies granted a license to the pharmaceutical company UCB to develop and commercialize therapies that target alpha-synuclein in all indications around the world (Click here to read the press release).
That agreement involved the development NPT200-11 (initially re-named UCB-1332 – Source), which entered Phase I testing in healthy volunteers in 2015 (Click here to read a press release related to this and click here to read more about that trial).
That Phase I study was completed in March 2015, and the companies announced that “NPT200-11 was well tolerated in 55 volunteers after oral administration” (Source).
The plan was then for NPT200-11 to enter clinical testing in September 2016 for Multiple Systems Atrophy (or MSA – an alpha synuclein-associated orphan disease that is similar to Parkinson’s – click here to read a previous SoPD post on MSA). I have not been able to find any evidence that the MSA study was ever started.
And at this point, my understanding (and I am happy to be corrected here) is that NPT200-11 was re-named UCB0599. And in May 2019, UCB initiated a Phase Ib clinical trial in Parkinson’s (Click here to read the press release).
And the results of that study were presented at the 2021 American Academy of Neurology virtual meeting last week.
|
# RECAP #2: Biotech firm Neuropore Therapies has developed molecules that reduce the aggregation of alpha synuclein. In collaboration with the pharmaceutical company UCB, they have been clinically testing one particular molecule NPT200-11/UCB0599. The results of a Phase Ib study have recently been presented. # |
What were the results of the clinical study?
In 2019, UCB initiated a US-based multicenter Phase Ib clinical trial of UCB0599 in Parkinson’s. 31 people with Parkinson’s (Hoehn-Yahr stage 1–3, aged 40–80 years) were recruited and they received two doses of either UCB0599 (n=21) or placebo (n=10) over four weeks (Click here to read the details of the trials). This was the first time the drug was tested in people with PD.
The results of the study indicate that UCB0599 was safe and well tolerated in the study. There was little difference between the UCB0599- and placebo-treated groups in terms of treatment-emergent adverse events (headache being the most common event in both groups).
The investigators reported that “UCB0599 was generally well tolerated with no significant safety or tolerability concerns identified in a population of older individuals with Parkinson’s with age-related comorbidity” (Click here to read the abstract)
The researchers concluded their presentation by stating that the data supports “further investigation of UCB0599 for the potential to slow PD progression“.
Have they started any further investigations of UCB0599?
Yes, they have.
In December 2020, UCB initiated the Phase IIa “ORCHESTRA” trial, which is a double-blind, placebo-controlled, randomized, 18-month study to assess the safety and tolerability of UCB0599 in 300 people with Parkinson’s.
The study will also be looking for any evidence that UCB0599 is superior to placebo in terms of slowing disease progression over 12 and 18 months (the primary outcome of the trial is MDS-UPDRS Parts I-III sum score).
The study is now recruiting individuals with early stage Parkinson’s at research sites in the U.S., Canada, and the Netherlands and the study is scheduled to finish in October 2023 (Click here to read more about this trial).
Is this the only small molecule targeting alpha synuclein in clinical trial?
No, definitely not.
There is a wide range of treatments being clinically developed that target alpha synuclein. From immunotherapy (which focuses on removing extracellular alpha synuclein – click here to read more about this) to drugs that prevent or break down the aggregation of alpha synuclein inside of cells (similar to UCB0599).
A good example of the later is a drug called anle138b which is being developed by the German biotech firm MODAG. Like UCB0599, anle138b is a molecule that inhibits the aggregation of alpha synuclein (Click here to read a previous SoPD post about this topic).
 In April 2020, MODAG announced that they had successfully completed Phase I testing of anle138b in healthy volunteers, and that the drug had “excellent safety and tolerability profiles at all dose levels and reached significantly higher plasma levels in humans than those required for full therapeutic efficacy in animal models” (Source).
In April 2020, MODAG announced that they had successfully completed Phase I testing of anle138b in healthy volunteers, and that the drug had “excellent safety and tolerability profiles at all dose levels and reached significantly higher plasma levels in humans than those required for full therapeutic efficacy in animal models” (Source).
In December 2020, the company initiated a Phase Ib clinical trial of anle138b in 24 individuals with mild to moderate Parkinson´s. This study is being conducted by Quotient Sciences in Nottingham (UK) with support from the Neurology Department of Nottingham University Hospital. The study will assess the safety, tolerability, and pharmacokinetics different doses of anle138b for seven days (Click here to read more about this study). This U.K. study is being supported by the Michael J. Fox Foundation and is scheduled to finish in June 2021.
Another example of an brain penetrant alpha synuclein targeting molecule called ANVS-401 (also known as Posiphen), which is being developed by the biotech firm Annovis (formerly QR Pharma).
 This company initiated a small Phase IIa trial in August 2020 evaluating various doses of this drug over 1 month of administration (Click here to read more about this). The study will recruit 14 people with PD and 14 with AD, and is mainly be exploring safety/tolerability, but there will also be some exploratory biomarker analysis. The study is scheduled to finish in September 2021 (Click here to read more about this).
This company initiated a small Phase IIa trial in August 2020 evaluating various doses of this drug over 1 month of administration (Click here to read more about this). The study will recruit 14 people with PD and 14 with AD, and is mainly be exploring safety/tolerability, but there will also be some exploratory biomarker analysis. The study is scheduled to finish in September 2021 (Click here to read more about this).
Is Neuropore Therapies working on any other alpha synuclein targeting molecules?
Interesting question, and the answer is yes.
The company is also working on a molecule called NPT520-34. Little is known about NPT520-34 (no preclinical data has been published), but according to the Neuropore Therapies website, it is a “orally bioavailable small molecule that reduces astrocytic and microglial markers of neuroinflammation with robust beneficial effects on neuropathology and motor function in animal models of Parkinson’s” (Source).
A presentation of NPT520-34 was made at the 2020 AAT-AD/PD Focus Meeting.
According to the presentation, NPT520-34 was discovered in a screening study of molecules that enhance the clearance of alpha synuclein in cells (via a waste disposal process called autophagy). In a genetically engineered alpha synuclein mouse model (L61), NPT520-34 treatment reduced “the accumulation of toxic proteins and markers of inflammation, preserved dopamine signaling in the brain, and improved grip strength and walking” (Source).
In May 2019, the company initiated a Phase I study that was “designed to evaluate the safety, tolerability and pharmacokinetics of NPT520-34” (Click here to read the press release and click here to read more about the details of this trial). In January 2020, Neuropore Therapies announced that the Phase I study was finished and the results “proved to be safe and tolerable at all doses tested, including those believed to be therapeutically relevant. The results of this study support moving forward to a safety study in patients” (Source).
We are now awaiting news regarding the next step in the development of NPT520-34.
So what does it all mean?
Alpha synuclein is widely viewed as one of the bad boy of Parkinson’s research. We have discussed it many times on this website. Previous attempts to target it in clinical trials have all focused on immunotherapy approaches, which boost the immune system and use it to remove alpha synuclein floating around outside of cells.
One potential issue with immunotherapy is that whole “outside of cells” part. Alpha synuclein aggregation is primarily perceived to be an intracellular phenomenon (that is, occurring inside of cells). Thus, it is extremely exciting that a selection of molecules is now reaching the stage of clinical development where we will be able to test whether reducing levels of aggregated alpha synuclein inside of cells could have any beneficial impact on disease progression. We have waited a long time for this.
One important question regarding this class of drugs is what happens to the dis-aggregated alpha synuclein? Does it get removed via waste disposal mechanisms? Or does it form other species of alpha synuclein (and if so, what are the consequences of that?)? Preclinical studies suggest that the treatments under clinical development are safe – reducing the likelihood that any kind of nasty species of the protein are being produced. But it would be interesting to know what happens to the dis-aggregated protein in humans treated with some of these agents under clinical development.
We should find out over the next few years the outcomes of some of these trials – expect SoPD posts as they get reported.
 All of the material on this website is licensed under a
All of the material on this website is licensed under a
Creative Commons Attribution 4.0 International License
You can do whatever you like with it!
The banner for today’s post was sourced from





