|
The new year has started with some pleasing clinical trial news for the Parkinson’s community: The results of the “Ambroxol in Disease Modification in Parkinson Disease” (AiM-PD study) have been published. This is a clinically available drug that is used for the treatment of respiratory issues, which researchers are re-purposing for Parkinson’s based on some interesting properties the drug has. The results of the clinical trial suggest that ambroxol was safe and well tolerated in people with Parkinson’s for the length of the 6 month study. It accessed the brain and increased levels of target proteins while there. In today’s post, we will discuss what ambroxol is, what research has been conducted on it, and what the results of this study suggest. |
The author of this blog is the deputy director of research at The Cure Parkinson’s Trust, and as such he feels that it is necessary to start this post with a very clear declaration – FULL DISCLOSURE: The Cure Parkinson’s Trust (in partnership with the Van Andel Institute) was a funder of the ambroxol clinical trial which is going to be discussed in this post.
Right. That said, let’s try and do a completely unbiased review of the ambroxol trial results ?
In one particular SoPD post last year we discussed the Linked Clinical Trials initiative, which is an international program that was set up 8 years ago with the goal of rapidly repurposing clinically available drugs exhibiting disease modifying potential in models of Parkinson’s (Click here to read the previous SoPD post on this topic).
What is meant by repurposing?
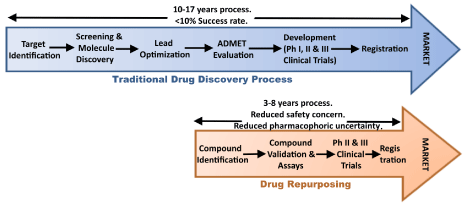
Drug repurposing (repositioning, reprofiling or re-tasking) is a strategy of identifying novel uses for clinically approved drugs that fall outside the scope of the original medical indication.
An example of this is “Viagra”.
It was originally developed as an anti-hypertensive medication, but was hugely more successful in the treatment of erectile dysfunction.
The strategy has been adopted and applied by many organisations because it allows for the by-passing of large parts of the drug discovery process, saving time and resources in getting new treatments to the clinic.
 Source: Austinpublishinggroup
Source: Austinpublishinggroup
By repurposing a clinically approved drug – for which we may know a great deal about already in terms of safety, tolerability and dose range – we can skip large parts of the clinical trial process and jump straight to testing the drug in our population of interest (in this case people with Parkinson’s).
And this is what the Linked Clinical Trials (or LCT) program was set up to do in Parkinson’s.
The first drug that was prioritised by the LCT committee for repurposing was a diabetes drug called exenatide (also known as Bydureon).
It is fair to say this LCT-initiated clinical trial program has provided interesting results thus far (Click here and here to read a SoPD post on this) and the exenatide program is now entering Phase III testing in Parkinson’s (Click here to read more about the Phase III trial).
In late 2014, the LCT committee prioritised another clinically available drug for repurposing to Parkinson’s.
That drug is called ambroxol.
What is ambroxol?
Ambroxol is a commonly used medication in Europe. It is used for the treatment for respiratory diseases (the respiratory system involving the lungs and related components required for breathing). Ambroxol promotes the clearance of mucus and eases coughing.
It also has anti-inflammatory properties, reducing redness in a sore throat.
 Ambroxol. Source: Wikipedia
Ambroxol. Source: Wikipedia
It is the active ingredient of products such as Mucosolvan, Mucobrox, and Mucol.
And why did the LCT committee think ambroxol could help with Parkinson’s?
Preclinical evidence (which we will discuss below) suggested that ambroxol could potentially help with Parkinson’s in two ways:
1. Ambroxol is believed to triggers exocytosis (Source). Exocytosis is the process by which waste is exported out of the cell (the opposite of ‘endocytosis’ which we discussed above). By encouraging exocytosis, ambroxol allows the cell to remove waste effectively and therefore function in a more normal fashion.

Exocytosis. Source: Socratic
2. Ambroxol has been shown to increase levels of an enzyme called glucocerebrosidase in the brain (Source).
Hang on a second. What exactly is gluco…cere…bro…si…dase?
Glucocerebrosidase (also known as GCase) is an enzyme that helps with the digestion and recycling of various proteins (particularly glucocerebrosides) inside cells.
The enzyme is located and active inside small bags of degradative enzymes. These bags are the lysosomes and they can be found floating around inside of cells.
How do these lysosomes work?
On a relatively continual basis, small parts of a cell membrane are being brought inside the cell. This is a process called endocytosis.
It occurs when the cell needs to consume resources from the outside world in order to find what it requires to function and survive. As a section of cell membrane is brought into the cell, it forms what is called a vesicle (which is a term used to refer to small spherical bags of stuff inside cells). Given the process by which these outer membrane vesicles are formed, they is referred to as endosomes (sometimes it is also called a vacuole).
 Source: Socratic
Source: Socratic
Once the endosome is inside the cell and detached from the rest of the membrane, it will bind to another vesicle which is called a lysosome. And as I mentioned above, lysosome is a small bag that is full of digestive enzymes, which help to break down the contents of the endosome.

How lysosomes work. Source: Prezi
The lysosome will fuse with the endosome/vacuole and the enzymes from the lysosome will mix with the material in the vacuole and digest it (or it break down into more manageable components).
This enzymatic process works in a very similar fashion to the commercial products that you use for washing your clothes.
 Enzymatic degradation. Source: Samvirke
Enzymatic degradation. Source: Samvirke
The reagents that you put into the washing machine with your clothes contain a multitude of enzymes, which help to break down the dirty, bacteria, flakes of skin, etc that cling to your clothes. Each enzyme breaks down a particular protein, fat or such like. And this is very similar to the collection of enzymes in the lysosome. All of them are needed to break down all of the contents of the endosome.
And if one of those enzymes – such as glucocerebrosidase – is faulty (due to a genetic mutation), then the enzymatic process is disrupted, which could result in the build up of un-degraded material over time.
|
RECAP #1: “Repurposing” of well chracterised, clinically available drugs is a method of speeding up the process of getting novel therapies to people with conditions like Parkinson’s. Ambroxol is a clinically available drug that is used in the treatment of respiratory conditions. Researchers are attempting to repurpose ambroxol for Parkinson’s because it is able to elevate levels of an enzyme called Glucocerebrosidase, which helps to boost waste disposal in cells.
|
Ok, but why would ambroxol possibly help people with Parkinson’s?
The enzyme GCase is produced by cells using instructions provided by the GBA gene (a gene is a section of DNA that provides the instructions for making a particular protein).
Genetic variations in the GBA gene are associated with Parkinson’s.
In fact, they are one of the most common genetic risk factors for the condition.
It is believed that approximately 5%–8% of people with Parkinson’s have a genetic mutation in their GBA gene (Click here and here to read more about this).
Interestingly, researchers have found that many people with Parkinson’s but without GBA mutations, have lower levels of GCase activity (Click here to read a previous SoPD post about this).
Researchers have been looking for drugs that can increase levels of GCase, in the hope that it will help boost the recycling system of cells of people with Parkinson’s and make the cells healthier. And if the cells are healthier, perhaps this would slow down the progression of Parkinson’s.
Thus, based on its ability to increase GCase levels, ambroxol represents a very interesting drug for repurposing to Parkinson’s.
So what do we know about ambroxol in models of Parkinson’s?
This is Prof Anthony Schapira:
 Source: Times-series
Source: Times-series
He is head of clinical neurosciences at the UCL Institute of Neurology and professor of neurology at the Royal Free hospital in London. And he has been driving much of the research on ambroxol in Parkinson’s.
In 2014, he and his team published this research report:

Title: Ambroxol improves lysosomal biochemistry in glucocerebrosidase mutation-linked Parkinson disease cells.
Authors: McNeill A, Magalhaes J, Shen C, Chau KY, Hughes D, Mehta A, Foltynie T, Cooper JM, Abramov AY, Gegg M, Schapira AH.
Journal: Brain. 2014 May;137(Pt 5):1481-95.
PMID: 24574503 (This report is OPEN ACCESS if you want to read it)
In this study, Prof Schapira and his team collected skin cells (called fibroblasts) from eleven people with GBA mutations (some of whom had been diagnosed with Parkinson’s). They measured the amount of GCase protein and enzyme activity in these cells, and they found that GCase enzyme activity was significantly reduced in fibroblasts from GBA mutations compared to normal fibroblasts (on average just the enzyme was acting at just 5% of normal levels). They found that ambroxol treatment increased GCase activity in fibroblasts from people with GBA mutations and in fibroblasts from healthy controls. Ambroxol treatment also reduced markers of oxidative stress in GBA mutant cells.
Given the role of GCase in the waste disposal system of cells, the researchers next wondered whether ambroxol treatment would reduce levels of alpha-synuclein in cells that were over-expressing this protein. Alpha synuclein is a protein that accumulates and clumps together in the brains of some people with Parkinson’s.
Amazingly, after 5 days of ambroxol treatment, levels of alpha-synuclein had decreased significantly (15% on average).
This result grabbed the researchers attention.
Here was a drug that not only re-activated the recycling unit in the cell, but also reduced levels of one of the main proteins associated with Parkinson’s. Ambroxol represented a potential candidate for repurposing.
And the results were supported by other independent research groups quickly finding similar results (Click here and here to read examples).
Interesting. What did the researchers do next?
Two years after that initial study, Prof Schapira and colleagues published this follow up study:

Title: Ambroxol effects in glucocerebrosidase and α-synuclein transgenic mice.
Authors: Migdalska-Richards A, Daly L, Bezard E, Schapira AH.
Journal: Ann Neurol. 2016 Nov;80(5):766-775.
PMID: 27859541 (This article is OPEN ACCESS if you would like to read it)
In this study, the researchers treated mice with ambroxol for 12 days and then measured the level of GCase activity in the brain. They gave ambroxol to three different groups of mice:
- a group of normal mice
- a group of mice which had been genetically engineered with a specific mutation in their GBA gene (the heterozygous L444P mutation)
- a group of mice that produced human alpha synuclein
When they looked at the level of GCase enzyme activity in normal mice, they found an increase of approximately 20% (in mice treated with 4mM ambroxol). One curious finding was that this dose was the only dose that increased GCase activity – 1, 3, and 5mM of ambroxol had limited effect. The investigators noted, however, a reduction in water drinking of mice receiving 5mM in their drinking water (maybe they didn’t like the taste of it!), suggesting that they were not getting as much ambroxol as the 4mM group.
The 4mM level of of ambroxol also increased GCase activity in the L444P mutation mice and the alpha-synuclein mice. One interesting observation was that alpha synuclein reduced levels of GCase activity.
But importantly, ambroxol treatment in the alpha synuclein mice was found to reduce the levels of alpha synuclein in the cells, indicating better clearance of un-wanted excess of proteins.
These combined results suggested to the investigators that not only was ambroxol entering the brain of mice (passing through the protective blood brain barrier – a membrane surrounding the brain), but it was able to be effective there. On top of this, the researchers did not witness any serious adverse effects of ambroxol administration in the mice – an observation also made in other studies of ambroxol in normal mice (Click here to read more about this).
And these studies were followed up by a dosing study in primates:

Title: Oral ambroxol increases brain glucocerebrosidase activity in a nonhuman primate.
Authors: Migdalska-Richards A, Ko WK, Li Q, Bezard E, Schapira AH.
Journal: Synapse. 2017 Mar 12. doi: 10.1002/syn.21967.
PMID: 28295625 (This article is OPEN ACCESS if you would like to read it)
In this study, the investigators analysed the effect of ambroxol treatment on Gcase activity in three healthy non-human primates. One subject was given an ineffective control solution vehicle, another subject received 22.5 mg/day of ambroxol and the third subject received 100 mg/day of ambroxol. They showed that daily administration 100 mg/day of ambroxol results in increased levels of GCase activity in the brain (approximately 20% increase on average across different areas of the brain). Importantly, the 22.5 mg treatment did not result in any increase.
The investigators also determined that 100 mg/day of ambroxol also increased HEXB activity (by approximately 20%), suggesting that ambroxol may be having an effect on other lysosome enzymes and not just GCase.
The researches concluded that ambroxol is active in the brain and “should be further investigated in the context of clinical trials as a potential treatment for Parkinson’s”.
Which brings us to the clinical trial.
|
RECAP #2: Preclinical research indicated that ambroxol can increase levels of the GCase enzyme in cells in culture, mice and primates. Those studies also found that ambroxol treatment could reduce the amount of the Parkinson’s-associated protein alpha synuclein. Based on these results, the researchers set up a clinical trial to test the safety of the drug in people with Parkinson’s.
|
So what happened in the clinical trial?
Between January 2017 and April 2018, a Phase II clinical trial “Ambroxol in Disease Modification in Parkinson Disease” (AiM-PD study) was conducted by Professor Schapira and his team (Click here to read more about the details of this study).
This week, the results of that study were published:
 Title: Ambroxol for the Treatment of Patients With Parkinson Disease With and Without Glucocerebrosidase Gene Mutations: A Nonrandomized, Noncontrolled Trial.
Title: Ambroxol for the Treatment of Patients With Parkinson Disease With and Without Glucocerebrosidase Gene Mutations: A Nonrandomized, Noncontrolled Trial.
Authors: Mullin S, Smith L, Lee K, D’Souza G, Woodgate P, Elflein J, Hällqvist J, Toffoli M, Streeter A, Hosking J, Heywood WE, Khengar R, Campbell P, Hehir J, Cable S, Mills K, Zetterberg H, Limousin P, Libri V, Foltynie T, Schapira AHV.
Journal: JAMA Neurol. 2020 Jan 13. [Epub ahead of print]
PMID: 31930374 (This report is OPEN ACCESS if you would like to read it)
In this study, the researchers were evaluating the safety and tolerability of ambroxol in 17 people with Parkinson’s. In addition, the investigators also assessed how well the drug accessed the brain and how much it increased levels of GCase. They also conducted some clinical assessments of disease progression, but given the open label nature of this study we won’t be putting much weight on that data.
What were the results of the study?
The AiM-PD study used a very high dose of ambroxol (1.26g/day or 21 pills per day) and found that it was well tolerated over the 6 month study. There were no reported serious adverse events due to the treatment during the study. The researchers also reported that the drug was entering the brain, and they recorded a 35% increase in GCase protein levels in the cerebrospinal fluid – this is the liquid surrounding the brain.
There was a 13% increase in alpha-synuclein concentrations in the cerebrospinal fluid, suggesting that more of this protein was being expelled from cells (via increased exocytosis).
The clinical assessments of the participants suggest that that treatment improved motor features associated with Parkinson’s (a 6.8 point reduction (indicating improvement) in the Movement Disorders Society unified Parkinson disease rating scale (MDS-UPDRS) Part III. Interestingly, these effects were observed in participants with and without GBA1 mutations.
But as I said above, this study was open label and not designed to evaluated disease modification. It was a very small study with no placebo control group. Thus, any interpretation of the clinical results should be taken with caution.
Interesting. So what happens next?
The Cure Parkinson’s Trust and the Van Andel Institute in collaboration with Prof Shapira and his team having been planning next steps in the ambroxol clinical trial program for the last 12 months, and unfortunately that is all I can say at present.
When concrete details are available (hopefully very shortly), I will share them with you here on the SoPD.
So what does it all mean?
The results of the AiM-PD study are very pleasing as they give us proof of concept that a Parkinson’s-associated protein can be targetted and increased in the brain using a clinically available treatment. But there is still a long way to go with this work. A larger and longer study is still required to determine if ambroxol can have any actual clinical benefits in slowing the progression of Parkinson’s.
And while the dose that was used in this study was well tolerated and found to be safe, it was also very high and required amazing stamina from the courageous participants (seriously, 21 pills per day! On top of their normal medication). Efforts have been made to reformulate the drug, but with limited success thus far.
The AiM-PD trial results represent another case study regarding the repurposing of drugs for Parkinson’s, joining exenatide as potential future treatment for the condition. And we will hopefully be hearing about other examples during 2020.

All of the material on this website is licensed under a
Creative Commons Attribution 4.0 International License
You can do whatever you like with it!
EDITOR’S NOTE: The information provided by the SoPD website is for information and educational purposes only. Under no circumstances should it ever be considered medical or actionable advice. It is provided by research scientists, not medical practitioners. Any actions taken – based on what has been read on the website – are the sole responsibility of the reader. Any actions being contemplated by readers should firstly be discussed with a qualified healthcare professional who is aware of your medical history. While some of the information discussed in this post may cause concern, please speak with your medical physician before attempting any change in an existing treatment regime.
Further, the author of this post is an employee of the Cure Parkinson’s Trust. The Trust has not asked for this post to be written, and there has been no effort to highlight the work of the Trust over others (perceptions of any bias should be directed to the author). This post has been written by the author solely for the purpose of sharing what the author considers interesting information.
The banner for today’s post was sourced from Skinflint.





