|
# # # # This week some encouraging clinical trial results were announced by a biotech firm called Anavex Life Sciences. The company had been testing their lead experimental therapy – a Sigma-1 receptor agonist called ANAVEX2-73 (also known as blarcamesine) – in 132 people with Parkinson’s disease dementia over a 14 week period. The results are rather encouraging: significantly positive outcomes in both cognitive and motor symptoms. In today’s post, we will explain what exactly “Sigma-1 receptor agonist” means, discuss what Parkinson’s disease dementia is, and review what we currently know about the results of the trial. # # # # |
 Source: Pumpingmarvellous
Source: Pumpingmarvellous
A lot of clinical trials for disease modification in Parkinson’s are focused on targeting well known proteins that are believed to be associated with underlying biology of the condition, such as alpha synuclein, LRRK2, and GBA. We discuss these on a regular basis here on the SoPD.
There are, however, a large number of trials investigating less well known targets.
And this week we received news that one of these clinical trials had some positive results.
 Source: Thestreet
Source: Thestreet
The study was conducted by the biotech company Anavex Life Sciences and it involved their lead experimental therapy ANAVEX2-73 (also known as blarcamesine).
ANAVEX2-73 is a Sigma-1 receptor agonist.
What does that mean?
An ‘agonist’ is a drug that binds to and activates a particular receptor.
Ok, but what is a receptor?
A receptor is a protein that act as switches for certain biological processes to be initiated. Receptors wait for a protein to come along and activate them or alternatively inhibit them. The activators are called agonists, while the inhibitors are antagonists.

Agonist vs antagonist. Source: Psychonautwiki
Cool. So ANAVEX2-73 activates a receptor. But what exactly is Sigma-1?
The Sigma-1 receptor is a multi-functional protein situated in endoplasmic reticulum membrane.
Que? What on Earth is the endoplasmic reticulum?!?
The endoplasmic reticulum (or ER) is a highly convoluted, netlike mesh structure that extends off the nucleus of a cell. It is the assembly line where proteins are produced within a cell.
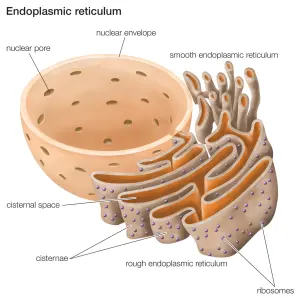 The endoplasmic reticulum. Source: Britannica
The endoplasmic reticulum. Source: Britannica
The nucleus is where the blue prints for making and maintaining an organism is kept in the form of DNA. A template of how to produce a particular protein can be generated from this DNA – that template is called RNA – and that is used to produce a protein (via a process called translation). A large part of that protein production process is conducted within the endoplasmic reticulum.
This video explains exactly what the ER does:
The Sigma-1 receptor is located in specific regions of the ER called the mitochondria-associated membrane. These are regions where mitochondria connect to the ER.
Mitochondria?
Mitochondria – you may recall from previous SoPD posts – are the power stations of each cell. They help to keep the lights on. Without them, the party is over and the cell dies.
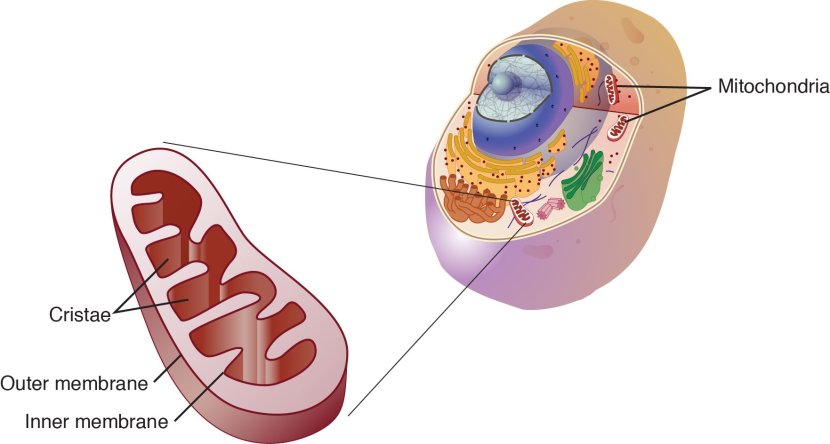
Mitochondria and their location in the cell. Source: NCBI
The mitochondria-associated membranes are involved in transferring certain molecules from the ER to mitochondria. They also play an important role in regulating calcium homeostasis, mitochondrial function, autophagy and apoptosis (don’t worry about all of that for now).
So what does the Sigma-1 receptor do?
Normally, the Sigma1 receptor is kept in check by a protein called Binding immunoglobulin protein (or BIP; also known as GRP78). When activated, however, Sigma-1 is released from the controlling grip of BIP, freeing Sigma-1 to interact with other proteins and molecular pathways, many of which have neuroprotective properties.
 Source: Semanticscholar
Source: Semanticscholar
This freedom is important for Sigma-1 because it functions as a chaperone.
What is a chaperone?
A chaperone is a protein that assist the folding (or unfolding) of other proteins. It helps other proteins to assemble into the right shape to do their function.
One protein Sigma-1 is a chaperone for is inositol 1,4,5-trisphosphate receptor (lets just call it IP3R). Sigma-1 helps IP3R to facilitate the flow of calcium from the ER under stressful conditions. Increasing Sigma-1 in cells helps to counteract ER stress, while lowering Sigma-1 levels results in more stress and leads to cell death (Click here to read more about this).
The chaperone role of Sigma-1 is important when a cell comes under stress. During ER stress, misfolded proteins start to pile up and accumulate as the normal protein producing function of the ER is interrupted. Activation of Sigma-1 aids in reversing this accumulation and restoring normal protein production (Click here to read more about this).
Sigma-1 can also activate the NF-κB-p65 pathway, resulting in increased levels of cellular antioxidants and decreased oxidative stress (Click here to read more about this). In addition to all of these actions, Sigma-1 may also promote synaptic plasticity and neuronal survival via increased levels of released BDNF (a neurotrophic factor, which promotes survival and growth of cells – click here to read more about this).
Given all of these functions, Sigma-1 is a really interesting target in Parkinson’s research.
|
# RECAP #1: Biotech company Anavex Life Sciences has an experimental drug called ANAVEX2-73, which is designed to activate the Sigma-1 receptor in cells. When activated the Sigma-1 receptor is free to facilitate antioxidant, neurotrophic, and neuroprotective pathways that could be useful in conditions like Parkinson’s. # |
What Parkinson’s-related research has been conducted on the Sigma-1 receptor?
So back in the mid 1990s, this study was published:
 Title: Sigma receptor-mediated neuroprotection against glutamate toxicity in primary rat neuronal cultures.
Title: Sigma receptor-mediated neuroprotection against glutamate toxicity in primary rat neuronal cultures.
Authors: DeCoster MA, Klette KL, Knight ES, Tortella FC.
Journal: Brain Res. 1995 Feb 6;671(1):45-53.
PMID: 7728532
In this study, the researchers evaluated the neuroprotective properties of 7 molecules that have the ability to activate Sigma-1. All of them were protective when administered to neurons in cell culture that had been over stimulated (excitotoxicity) by a neurotransmitter called glutamate.
It was one of the first reports suggesting a neuroprotective role for Sigma-1.
And this neuroprotective effect of sigma-1 activation was quickly replicated in dopamine neurons (a population of cells that are badly affected in Parkinson’s) that had been grown in cell culture (Click here to read more about that research).
These cell culture results have been replicated numerous times, including in mouse models of Parkinson’s. For example, this recent report:
 Title: Development and characterization of an inducible Dicer conditional knockout mouse model of Parkinson’s disease: validation of the antiparkinsonian effects of a sigma-1 receptor agonist and dihydromyricetin.
Title: Development and characterization of an inducible Dicer conditional knockout mouse model of Parkinson’s disease: validation of the antiparkinsonian effects of a sigma-1 receptor agonist and dihydromyricetin.
Authors: Guo CH, Cao T, Zheng LT, Waddington JL, Zhen XC.
Journal: Acta Pharmacol Sin. 2020 Apr;41(4):499-507.
PMID: 32112040 (This report is OPEN ACCESS if you would like to read it)
In this study, the researchers used genetically engineered mice (called Dicer knockout mice) that gradually lose their dopamine neurons over time after being treated with a drug called tamoxifen.
The investigators treated these mice with a Sigma-1 receptor agonist called PRE-084 and observed not only a complete rescue of the dopamine neurons (as measured in number of TH-positive neurons), but also a significant reduction in inflammation in the brain (as measured in number of Iba-1-positive cells):
 Source: PMC
Source: PMC
And this neuroprotection may be associated with a restorative effect. Evidence supporting this idea was published in 2019:
 Title: Pridopidine Induces Functional Neurorestoration Via the Sigma-1 Receptor in a Mouse Model of Parkinson’s Disease.
Title: Pridopidine Induces Functional Neurorestoration Via the Sigma-1 Receptor in a Mouse Model of Parkinson’s Disease.
Authors: Francardo V, Geva M, Bez F, Denis Q, Steiner L, Hayden MR, Cenci MA.
Journal: Neurotherapeutics. 2019 Apr;16(2):465-479.
PMID: 30756361 (This report is OPEN ACCESS if you would like to read it)
In this study, the researchers took mice that had been injected with a neurotoxin in one side of their brain. The neurotoxin (called 6-OHDA) kills only dopamine neurons and has been used as a method of modelling Parkinson’s for a long time. The investigators treated these mice with either a sigma-1 agonist (called pridopidine) or a placebo treatment every day for 5 weeks. Two different doses of the agonist were tested (a high dose and a low dose).
The investigators reported that the low dose significantly improved behavioural deficits in the mice and exhibited increased dopamine fiber density in the striatum (in the image below dark staining represents the branches of dopamine neurons in a region of the mouse brain called the striatum – the more dopamine branches, the more dark staining. The top row represents an image of a section of the striatum for placebo treated (saline), low dose (Pridop 0.3), and high dose (Pridop 1) treated mice. The bottom row represents high magnification images of the small black square areas in the images above (right hand side of each image). As you can hopefully see the images in the middle column (low dose) have a lot more dark staining – indicating more dopamine branches – than the images on the left or right:
 Source: PMC
Source: PMC
This result suggested that not only was activation of Sigma-1 potentially neuroprotective, but also possibly restorative. And the effect was specific to Sigma-1, because the positive results of low dose pridopidine were absent in 6-OHDA-lesioned mice that had been genetically engineered to have no Sigma-1 receptor.
Like I said, Sigma-1 is a really interesting target in Parkinson’s research.
|
# # RECAP #2: Preclinical research in models of neurodegeneration indicates that activation of the Sigma-1 receptor is neuroprotective. Studies in rodent models of Parkinson’s have expanded on this observation, and provided some evidence that Sigma-1 could also be restorative. # # |
So what were the ‘encouraging’ results of the clinical trial?
The trial was a Phase 2, double-blind, randomized, placebo-controlled study to evaluate the safety, tolerability, and efficacy of ANAVEX2-73/Blarcamesine on cognitive impairment in 132 individuals with Parkinson’s Disease Dementia (PDD).
What is Parkinson’s Disease Dementia?
Parkinson’s Disease Dementia (PDD) is very similar to Parkinson’s in that the sufferer will have the characteristic features of the condition (rigidity, slowness of movement, and a resting tremor), but a marked decline in thinking, problem-solving and memory will develop over time.
There are two types of dementia associated with PD, and they are divided into two conditions, based on timing (Source):
- Dementia with Lewy bodies (DLB) – cognitive symptoms develop within a year of parkinsonism
- Parkinson’s disease dementia (PDD) – cognitive symptoms develop more than a year after the onset of movement symptoms
Both DLB and PDD share similar “underlying changes in the brain and very similar symptoms, but the symptoms appear in a different order” (The Davis Phinney Foundation have a great webpage on this topic – click here to read it).
Ok, so what did the Anavex trial involve?
The participants in the trial were recruited at multiple sites in Spain and Australia, and randomised (ratio of 1:1:1) to either a placebo treatment, 30mg, or 50mg of ANAVEX2-73 per day for 14 weeks. They were then followed over time with various clinical assessments. Study participants were allowed to be on stable regimen of anti-Parkinson’s disease medications, and required a diagnosis of probable PDD (based on the MDS Task Force clinical diagnostic criteria as well as a Montreal Cognitive Assessment (MoCA) score of 13 to 23).
The primary endpoint of the study (which is a predetermined measure of the efficacy of a treatment) was performance on the Cognitive Drug Research (CDR) Computerized Assessment System Continuity of Attention – a computer-based battery of cognitive tests. The participants were tested at baseline and then again after 14 weeks of treatment. The secondary endpoint was determined by the Movement Disorder Society- Unified Parkinson Disease Rating Scale (or MDS-UPDRS – Click here to read more about the details of this trial).
And what were the results of the study?
According to their press release, Anavex has reported that 14 weeks of ANAVEX2-73 treatment resulted in a significant increase in levels of the SIGMAR1 mRNA in blood samples collected of participants. This biomarker increase was associated with significant improvements in CDR system measures (assessed by Choice Reaction Time (p = 0.039) and Digital Vigilance (p = 0.008) and CDR system Episodic Memory (p = 0.047), representing complex cognitive tasks).
What about the Parkinson’s symptoms?
So this is where it gets really interesting.
The press release reads: “From baseline to end of trial at 14 weeks, MDS-UPDRS Total score improved by -10.98 points in the ANAVEX®2-73 high dose group and worsened by 3.53 points in the placebo group, an adjusted mean difference of -14.51 points (p = 0.034).”
This is rather jaw dropping stuff.
A 14-week study is very short, and an improvement of 11 points is simply remarkable. It corresponds to a relative improvement of almost 20% over 14 weeks (18.9% to be exact).
And it is important to remember that is that this result was achieved while the participants were on their standard medication. That is to say, the participants were on their standard PD medication (e.g. L-dopa, etc) during the clinical assessments – so the improvement in total UPDRS score was on top of the response to standard medication.
Typically, individuals with PD decline in MDS-UPDRS scores by 3.99 to 7.45 points per year (Click here to read more about this), so a 10.98 point improvement is worth noting (remember this was a double-blinded study).
Wow! Too good to be true?
We need to see more data. Press releases are great, but they are limited in how much information that offer.
Important to note that the press release only discusses the results of the participants treated with the high dose (50mg) of ANAVEX®2-73. Plus such a rapid and dramatic change could simply be a symptomatic response (not a disease modifying change).
How do you explain the improvement in the cognitive assessments then?
Like I said, we need to see more data. Without it, we would be simply speculating.
In addition, the press release mentions improvements in other areas, such as sleep (the press release reads: “ANAVEX®2-73 does not impair sleep and has a positive effect on REM sleep behavior disorder“). Better sleep could potentially have an impact on cognitive and motor performance.
But it is certainly an encouraging result.
After completing the trial, the participants had the option of continuing to be treated with ANAVEX2-73 for nearly 12 months in an extension study, called ANAVEX 2-73-PDD-EP-001. That extension is expected to conclude in October so there may be more data coming later this year or early next year (Click here to read more about the details of this study).
What are Anavex planning to do next?
At present, this is not clear. In the press release, Anavex Life Science says that they are planning to present their data at an upcoming research conference later this year. In addition, they are seeking to submit the result to the U.S. Food and Drug Administration in order to gain regulatory guidance for a future Phase 3 trial.
The company also has a Michael J. Fox Foundation funded imaging study starting soon. The study will “explore utilization of PET imaging biomarkers to enable measurement of target engagement and pathway activation of the sigma-1 receptor with clinically relevant doses in people with Parkinson’s” (Click here to learn more about this).
Given the number of biotech companies that were acquired by pharma companies last year, it would not be surprising if one of the big companies step in and buy Anavex – especially given that the company announced positive results in another clinical trial of ANAVEX2-73 in Rett syndrome – a rare genetic disorder that affects brain development (Click here and here to read more about this).
Is Anavex the only company developing a sigma-1 receptor agonist?
No.
Prilenia Therapeutics are currently clinically testing the sigma-1 receptor agonist called Pridopidine (which was mentioned in one of the research reports discussed above). Pridopidine (previously known as ACR16 or Huntexil) is currently being tested in a Phase III clinical trial for Huntington’s disease (Click here to read more about this), but it is also being evaluated as a treatment for Levodopa-induced dyskinesias in Parkinson’s. The study involves 12 weeks of treatment in 135 participants (Click here to read more about this trial).
Pridopidine (previously known as ACR16 or Huntexil) is currently being tested in a Phase III clinical trial for Huntington’s disease (Click here to read more about this), but it is also being evaluated as a treatment for Levodopa-induced dyskinesias in Parkinson’s. The study involves 12 weeks of treatment in 135 participants (Click here to read more about this trial).
And pridopidine has been tested in models of Parkinson’s – see the Francardo et al (2019) report mentioned in this post.
It should also be noted that Anavex Life Science has been developing two additional Sigma-1 receptor agonists – ANAVEX3-71 and ANAVEX1-41 – which are both indicated for neurodegenerative conditions on the company’s website (Click here to read more about these).
ANAVEX3-71 has recently been tested in a Phase I clinical trial (Click here to read more about this), so it will be interesting to see which direction that take this molecule.
So what does it all mean?
In the wake of the ridiculous FDA approval of a new therapy for Alzheimer’s for which there was so little actual supporting data, it is refreshing to see some encouraging results coming from the field of Parkinson’s research. An actual positive result on both the primary and secondary endpoints in the Anavex Phase 2 study of Parkinson’s disease dementia deserves more attention than it has received in the media.
Having said that, there is still work to be done before ANAVEX2-73/Blarcamesine can be approved for clinical use. A Phase III trial demonstrating efficacy and safety in a larger PD cohort over a longer period of time is needed. In addition, it would be useful to know the possible mechanisms of action by which the therapy is having its effect.
In the post-truth, aducanumab world that we now live in, it will be interesting to see what kind of guidance the US FDA gives Anavex Life Science. Hopefully some form of acceleration program will allow for rapid progress for this potential therapy.
 All of the material on this website is licensed under a
All of the material on this website is licensed under a
Creative Commons Attribution 4.0 International License
You can do whatever you like with it!
EDITOR’S NOTE: Anavex Life Science Ltd is a publicly traded company. The material presented on this page should under no circumstances be considered financial advice. Any actions taken by the reader based on reading this material is the sole responsibility of the reader. Anavex have not requested that this material be produced, nor has the author had any contact with the company or associated parties (except to ask for a copy of the report reviewed. This post has been produced for educational purposes only.
The banner for today’s post was sourced from Anavex


