|
# # # # On the 8th June, BlueRock Therapeutics put out a press release announcing that the first participant in their Phase I clinical trial of cell transplantation for Parkinson’s had been dosed (Click here to read the press release). The initiation of this clinical trial by the company is a major step forward for them and for the wider field of regenerative therapies. In today’s post, we will look at what cell transplantation is, recent developments in clinical trials, and what the immediate future holds. # # # # |
 Source: The Scientist
Source: The Scientist
Here on the SoPD, we work around the idea that any “curative therapy” for Parkinson’s is going to require three core components:
- A disease halting mechanism
- A neuroprotective agent
- Some form of restorative therapy
Parkinson’s is a progressive neurodegenerative condition, meaning that symptoms are gradually going to get worse over time. Thus, the first and most critical component of any ‘cure’ for Parkinson’s involves a treatment that will slow down or halt the progression of the condition.
Once such a therapy has been identified, it will be necessary to rejuvenate and protect the remaining cells. So, some form of neuroprotective therapy that can help bring sick or dying cells back to life will be required.
Such a treatment will also provide a nurturing environment for the third part of the ‘cure’: A restorative treatment. New cells will be required to replace the lost function.
Now, the bad news is (as far as I am aware) there is no single treatment currently available (or being tested) that can do all three of these things. By this I mean that there is no “disease halting mechanism” therapy that can also replace lost brain cells. Nor is there a restorative therapy that stop the progression of the condition.
That statement can obviously be read as terrible news, but it shouldn’t.
Let me explain:
Any “curative therapy” for Parkinson’s is going to need to be personalised to each individual, with varying levels of each of the three component listed above. It will need to be a multi-modal approach designed for each individual’s needs.
 Making things personal. Source: Flickr
Making things personal. Source: Flickr
There is a great deal of heterogeneity (or variability) between individuals with regards to their symptoms, their progression, and the amount of time that they have had the condition. Some folks are more tremor dominant, while others do not experience tremor at all. Likewise, some individuals have only just been diagnosed, while others have lived with the condition for many years.
Given this level of variability, it may be that Parkinson’s is not a single condition, but rather a collection of similar looking conditions, with different causal factors. Regardless, the premise stands: Treatments need to be personalised.
The treatment needs of each individual will be different, and we will ultimately require different amounts of the disease halting mechanism component, the neuroprotection component, and the restorative therapy components for each affected person.
In today’s post, we will look at some updates and new developments in research on the third component outlined above: A restorative treatment.
What kind of restorative treatments are being developed for Parkinson’s?
At present, most of the clinical trials are focused on cell replacement or cell transplantation therapies.
What does cell transplantation therapy involve?
By the time a person is presenting the motor features characteristic of Parkinson’s, and being referred to a neurologist for diagnosis, they have already lost approximately 50% of the dopamine producing neurons in an area of the brain called the midbrain. The dopamine neurons are located in a sub-region of midbrain called the substantia nigra.
 The dark pigmented dopamine neurons in the substantia nigra are reduced in the Parkinsonian brain (right). Source:Memorangapp
The dark pigmented dopamine neurons in the substantia nigra are reduced in the Parkinsonian brain (right). Source:Memorangapp
These cells are critical for normal motor function – without them, movement becomes very inhibited, resulting in the slowness and rigidity associated with the condition.
And until we have developed methods that can identify Parkinson’s long before these cells are lost and the motor features appear, some form of cell replacement therapy is required to introduce new cells to take up the lost function.
Cell transplantation represents the most straight forward (but still experimental) method of cell replacement therapy.
How does cell transplantation work?
This traditional approach to cell transplantation has involved dissecting out the region of the developing dopamine neurons from a donor embryo, breaking up the tissue into small pieces that could be passed through a tiny syringe, and then injecting those cells into the brain of a person with Parkinson’s.

The old cell transplantation process for Parkinson’s. Source: The Lancet
Critically, the people receiving this sort of transplant have required ‘immunosuppression treatment’ for a long period of time after the surgery. This additional treatment involves taking drugs that suppress the immune system’s ability to defend the body from foreign agents. This step is necessary, however, in order to stop the body’s immune system from attacking the transplanted cells (which would not be considered ‘self’ by the immune system), allowing those cells to have time to mature, integrate into the brain and produce dopamine.
The transplanted cells have typically not been injected into the midbrain (where the cells are lost) but rather an area of the brain called the putamen.
What is the putamen?
As mentioned above, the bulk of the dopamine neurons in the brain reside in the substantia nigra, but they project their branches (or axons) to the several other areas, including the putamen, and this is where they release most of their actual dopamine (the chemical which helps us to move properly).
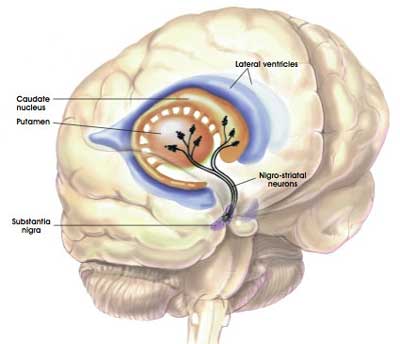 The projections of the substantia nigra dopamine neurons & location of the putamen. Source: MyBrainNotes
The projections of the substantia nigra dopamine neurons & location of the putamen. Source: MyBrainNotes
In people with Parkinson’s, the amount of dopamine being released in the putamen decreases over time. The image below demonstrates the loss of dopamine-releasing fibres (the dark staining) over time as a result of Parkinson’s (PLEASE NOTE that the time scale presented here varies from person to person):
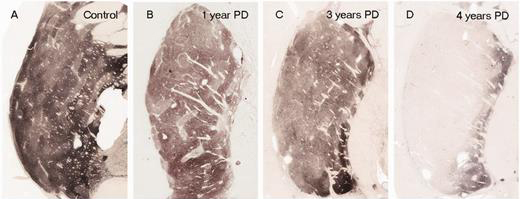
The loss of dopamine in the putamen as Parkinson’s progresses. Source: Brain
In cell transplant procedures for Parkinson’s, multiple injections of cells are usually made in the putamen, allowing for deposits in different areas of the structure. These multiple sites allow for the transplanted cells to produce dopamine across the entire extent of the putamen. And ideally, the cells should remain localised to the putamen, so that they are not producing dopamine in areas of the brain where it is not desired (possibly leading to side effects).

Targeting transplants into the putamen. Source: Intechopen
Postmortem analysis of the brains of individuals who have previously received transplants of dopamine neurons (and then subsequently died from natural causes) has revealed that the transplanted cells can survive the surgical procedure and integrate into the host brain – in some cases producing dopamine for decades post transplantation.
In the image below, you can see rich brown areas of the putamen in panel A. These brown areas are the dopamine producing cells. A magnified image of individual dopamine producing neurons (their circular bodies and their branches are stained in brown) can be seen in panel B:

Transplanted dopamine neurons. Source: Sciencedirect
The transplanted cells take several years to develop into mature neurons after the transplantation surgery – the same way they develop and mature in the brain of an infant child. This means that the actually benefits of the transplantation technique will not be apparent for some time (2-3 years on average). Once mature, however, it has also been demonstrated (using brain imaging techniques) that these transplanted cells can produce dopamine.
As you can see in the images below, there is less dopamine being processed (indicated in red) in the putamen of the Parkinsonian brain on the left than the brain on the right (several years after bilateral – both sides of the brain – transplants):

Brain imaging of dopamine processing before and after a successful transplantation. Source: NIH
In some cases the individual being transplanted has been able to reduce the amount of Levodopa treatment that they take over time. As the transplanted cells start to produce enough dopamine in the right area of the brain, it allows the individual to function better and require less medication.
|
# RECAP #1: Cell transplantation is an experimental approach for restorative therapy in Parkinson’s. It involves injecting new cells into the brain, in the hope of restoring lost function. Typically this approach has involved injected new dopamine producing cells into an area of the brain called the putamen, where the bulk of the dopamine in the brain is released. # |
This sounds great! Like a cure for Parkinson’s?
Yeah, not really.
As we discussed above, restorative therapies are not curative (in the absense of a disease halting treatment). It may help to replace the dopamine producing cells, but Parkinson’s will continue progressing under the surface of this.
In addition, the history of clinical trials for cell transplantation in Parkinson’s has been a bit of a rollercoaster ride. There have been some success stories and there have also been some not-so-successful stories. The main issue to date has been the type of tissue being transplanted and the variability in the quality of the tissue between studies.
This situation has changed recently, however, as researchers have shifted away from the old approach to cell transplantation (using dopamine neurons from a donor embryo), and moving towards developing sophisticated recipes for growing dopamine neurons in cell culture.
Researchers can now grow millions of dopamine neurons in petri dishes, which provides more than enough cells for transplanting numerous individuals with Parkinson’s.
But it is important to understand that – by itself – cell transplantation is not a cure for Parkinson’s. Based on our current understanding, cell transplantation will not halt the progression of the condition. It is simply an experimental method of replacing lost cells. It is hoped that this treatment approach will eventually develop to the point where it will allow individuals affected by the condition to reduce their dopamine-based treatment over time. But as far as we can tell the procedure does not slow or stop the underlying mechanisms driving the neurodegeneration associated with Parkinson’s. In addition, the procedure is unlikely to affect non-motor aspects of the disease.
So what new developments are there?
The most recent (last month) development has been the announcement by a biotech company called BlueRock Therapeutics that the first participant in their Phase I clinical trial of cell transplantation for Parkinson’s had been treated (Click here to read the press release).
What is BlueRock Therapeutics?
BlueRock is a biotech firm that was foundered in 2016 to focus on developing engineered cell therapies in the fields of neurology, cardiology and immunology, using a proprietary iPS cell platform.
What are iPS cells?
iPS stands for “Induced pluripotent stem” (cells).
Pluripotent stem cells are cells that can be grown (in cell culture) to become any type of cell in the body – heart, brain, bone, blood, etc. ‘Pluripotent’ meaning capable of any fate. These cells start off in a naïve state, but using specific protocols (or recipes) of certain proteins, these cells can be encouraged to differentiate into any kind of cell we may require.
 The menu. Source: Wikipedia
The menu. Source: Wikipedia
But it is the “induced” part of their name that makes iPS cells really special.
This is Prof Shinya Yamanaka:

Prof Shinya Yamanaka. Source: Glastone Institute
He’s a rockstar in the biomedical research community.
Prof Yamanaka is the director of Center for induced Pluripotent Stem Cell Research and Application (CiRA); and a professor at the Institute for Frontier Medical Sciences at Kyoto University.
But more importantly, in 2006 he published a research report that would quite literally ‘change everything’.
This is the report:

Title: Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors.
Authors: Takahashi K, Yamanaka S.
Journal: Cell. 2006 Aug 25;126(4):663-76.
PMID: 16904174 (This article is OPEN ACCESS if you would like to read it)
In this study, Prof Yamanaka‘s team demonstrated a method by which someone could take a simple skin cell (called a fibroblast), grow it in cell culture for a while, and then re-program it so that it would transform back into a stem cell – a cell that is capable of becoming any kind of cell in the body.
So rather than just being a skin cell that could divide into other skins cells, the transformed (or “induced”) ‘stem cell’ could now become any kind of cell. It was pluripotent.
And because they had persuaded the skin cells to change back to this immature state, the researchers decided to call the transformed cells induced pluripotent stem (or IPS) cells.
Prof Yamanaka‘s team started their investigation with the basic hypothesis that proteins which are important to the maintenance of embryonic stem cells (the cells that give rise to all of the cells in your body) might also be able to cause an embryonic state in mature adult cells. They selected twenty-four proteins that had been previously identified as important in embryonic stem cells to test this idea. They used re-engineered viruses to deliver these proteins to mouse skin cells. The viruses were emptied of all their disease causing properties, and could thus function as very efficient biological delivery systems.
The skin cells were genetically engineered in such a fashion so that only cells in which reactivation of the embryonic stem cells-associated protein, Fbx15, would survive the testing process. If Fbx15 was not turned on in the cells, over time they would die. When the researchers infected the cells with all twenty-four embryonic stem cells genes, remarkably some of the cells actually survived and began to divide like stem cells.
In order to identify which proteins were necessary for the reprogramming, the researchers began removing one protein at a time from the pool of twenty-four. Through this process, they were able to narrow down the most effective proteins to just four: Oct4, Sox2, cMyc, and Klf4, which became known as the “Yamanaka factors”.
Interesting. But why is this important?
Understand that this was more than just an amazing feat of molecular biology. It suddenly made the hypothetical idea of ‘personalised medicine’ very possible – take skin cells from anyone with a particular medical condition, turn them into whatever cell type you like, and then either test drugs on those cells or transplant them back into their body (replacing the cells that have been lost due to the medical condition).
Personalised medicine with IPS cells. Source: Bodyhacks
Ok, so Bluerock is using these iPS cells for cell therapies?
Yes, and very recently they announced that they had initiated a Phase I clinical trial of their iPS cell derived dopamine neuron product, which is being called DA01. In early June, the company put out a press release stating that the first participant in their study had been transplanted (Click here to read the press release).
The trial will be very small – enrolling just 10 patients at research sites in the US and Canada. The participants will undergo surgical transplantation of the DA01 dopamine-producing cells into their putamen, and then be followed over the next 2 years – the results of the study will hopefully be available in early 2024 (Click here to read more about the details of this trial).
This is good news for the “Bluerockers” who have been trying to get this clinical trial started for several years. It is also good news for their owner – the pharmaceutical company Bayer.
In August 2019, Bayer announced that they would “fully acquire BlueRock Therapeutics, a privately held US-headquartered biotechnology company focused on developing engineered cell therapies in the fields of neurology, cardiology and immunology, using a proprietary induced pluripotent stem cell (iPSC) platform” (Source).
|
# # RECAP #2: Induced pluripotent stem (or IPS) cells are a new type of tissue that opens the door for a more personalised forms of medicine. By taking skin cells from patients and applying molecular biology techniques to those cells, any type of cell can be generated – hopefully reducing the need for immunosuppression medication in cell transplantation procedures. IPS cells are now being applied to Parkinson’s, with encouraging preclinical data in models of PD leading to clinical trials being initiated around the world. # # |
So what else is happening in the world of cell transplantation for Parkinson’s?
Quite a lot actually.
There are a number of ongoing clinical trial for cell transplantation in Parkinson’s. Firstly, there is the TRANSEURO study which is using the traditional fetal cell approach to develop new protocols for novel methods of cell replacement therapy.
This trial is an open label study, involving 13 subjects. This study is scheduled to finish in 2021 (Click here to read more about this).
There are also a number of trials evaluating approaches involving the expansion of stem cells in cell culture, partially maturing the cells towards a dopamine neuron fate before transplantation. The first of these is a Phase 1 clinical trial being conducted in Kyoto, Japan.
The ongoing Kyoto cell transplantation trial is being conducted by researchers at the Center for iPS Cell Research and Application (or CiRA).
 In 2017, the researchers behind this trial published a report evaluating their IPS cells in primate models of Parkinson’s (Click here to read more about that research report).
In 2017, the researchers behind this trial published a report evaluating their IPS cells in primate models of Parkinson’s (Click here to read more about that research report).
Information provided by Kyoto University (Click here for the study website) and the Japanese media has indicated that this Phase I/II clinical trial aims to investigate the safety and efficacy of transplanting human IPS cell-derived dopaminergic progenitors into the brains of people with Parkinson’s (what stage of PD has not been disclosed). The study is very small, involving just 7 participants – who are all Japanese individuals with Parkinson’s. The participants in the study will be followed and assessed for 2 years post transplantation
According to the CIRA 2018 annual report, the researchers began enrolling patients on 1st August and the first patient was transplanted in October (2018 – Click here to read the annual review). The investigators stated that the gentleman who was transplanted will be monitored for 2 years.
The team leading the Kyoto IPS-cell derived transplantation trial for Parkinson’s provided an update late last year. The team said that none of the three transplanted patients who have been transplanted developed any side effects (>12 months post surgery). In addition, they were planning 4 more transplants going ahead (I’m not sure how much COVID-19 may have slowed that progress down).
The Kyoto researchers are also working very closely with the pharmaceutical company Sumitomo Dainippon Pharma, which announced in March of this year that they were planning to begin US based clinical trials in 2022 of the iPS-derived cell approach for cell transplantation in Parkinson’s (Click here to read more about this).
Sumitomo has been working on better manufacturing and selection techniques for iPS cell-derived dopaminergic progenitor cells (Click here to read more about this).
The second stem cell-based cell transplantation clinical trial was initiated in 2018 by a research group from China led by Professor Qi Zhou, a stem-cell specialist at the Chinese Academy of Sciences Institute of Zoology.
This study is taking place at The First Affiliated Hospital of Zhengzhou University in Henan province, and involves 10 participants being injected with neuronal-precursor cells derived from embryonic stem cells (Click here to read more about this trial). The study was scheduled to finish in late 2020, but we are yet to hear any news regarding this study (Click here to read a previous SoPD post on this topic).
A third trial is being conducted by Allife Medical Science and Technology Co. in China.
 This study of 10 participants is also a safety trial, it is also using neuronal-precursor cells derived from embryonic stem cells, and it is scheduled to finish in 2021 (Click here to read more about this study).
This study of 10 participants is also a safety trial, it is also using neuronal-precursor cells derived from embryonic stem cells, and it is scheduled to finish in 2021 (Click here to read more about this study).
There has also been a clinical study conducted by International Stem Cell Corp (ISCO) in Australia. The company recently announced the completion of that study and that the treatment was safe (Click here to read more about this). Here at the SoPD we have been very concerned about this trial (Click here and here to read previous posts about this), so it is reassuring to learn of this outcome.
One biotech company focused on cell transplantation for Parkinson’s that we have a lot more confidence in is Aspen Neuroscience.
 This company has been developing two products: ANPD001 – an autologous neuron replacement therapy in Parkinson’s, and a second product ANPD002 which is “a gene-edited autologous neuron replacement therapy for familial forms of Parkinson’s” which appears to be initially focused on GBA-associated Parkinson’s (Source).
This company has been developing two products: ANPD001 – an autologous neuron replacement therapy in Parkinson’s, and a second product ANPD002 which is “a gene-edited autologous neuron replacement therapy for familial forms of Parkinson’s” which appears to be initially focused on GBA-associated Parkinson’s (Source).
In April 2020, Aspen announced that they had raised US$70 million in Series A funding to help progress development of their program (Click here to read a SoPD post on this topic). They are keen to start clinical testing in the very near future.
In addition to these transplantation programs, there are a number of parties exploring how to further develop their own research in this area.
There was recently an “N=1” cell transplantation study that was published in the New England Journal of Medicine (Click here to read a previous SoPD post about this), and it would appear that the research group behind that study are trying to develop their cell transplantation approach further via the biotech company NurrOn Pharmaceuticals.
Earlier this year, the researchers behind a biotech company called BrainXell published data from cell transplant studies in non-human primate models of Parkinson’s (Click here to read more about that), so we will hopefully learn more soon about their plans going forward towards the clinic.
One biotech firm in the field of stem cell biology that we have been keeping an eye on at the SoPD is Fujifilm/Cellular Dynamics.
They have a very active cell transplantation program under preclinical development for Parkinson’s (Source), and we have been quietly waiting for any news announcing a partnership for taking their cells forward into a clinical trials exploring cell transplantation in Parkinson’s.
 Source: Fujifilm
Source: Fujifilm
And finally, in a fascinating presentation at the World Parkinson’s Coalition’s virtual meeting in May this year, Dr Agnete Kirkeby provided some of the details regarding the STEM-PD clinical trial that will be starting in 2022.
This will be a collaboration between the University of Lund, Cambridge University and the pharmaceutical company Novo Nordisk (Source).
 The details provided during the presentation indicate that the STEM-PD trial will be small (just 8 patients) with a primary endpoint of safety (determined at 12 months post transplant) and a secondary endpoint of efficacy (measured at 3 years post transplant). The participants will have been diagnosed more than 5 years ago, with mild/moderate symptoms, and they will receive one of two doses.
The details provided during the presentation indicate that the STEM-PD trial will be small (just 8 patients) with a primary endpoint of safety (determined at 12 months post transplant) and a secondary endpoint of efficacy (measured at 3 years post transplant). The participants will have been diagnosed more than 5 years ago, with mild/moderate symptoms, and they will receive one of two doses.
Some of these details may change before the trial officially starts (hopefully in 2022 as indicated in the presentation), but it is very encouraging to see industry and academic collaboration in this endeavour.
So to answer your question, it is safe to say that there is a lot happening in the world of cell transplantation for Parkinson’s (apologies if I left anyone out – contact me here if I have).
So what does it all mean?
Replacing some of the cells that are lost in Parkinson’s is desired goal for many affected by the condition. But it is important to remember that cell transplantation is still very experimental. Anyone selling a cell transplant product for Parkinson’s should be avoided as there are currently no clinically approved methods. Reliance on testimonials is not an appropriate measure of success, and in most cases the tissue being transplanted has not well researched (and published in publicly available peer-reviewed journals). While I appreciate the desperate desire expressed by many in the PD community to explore this path, the SoPD does not condone it – we have heard too many horror stories.
The initiation of the BlueRock therapeutics trial is good news for the field of cell transplantation research in Parkinson’s. It represents a major milestone for the company, whose founders have been working on their technology for over a decade (congrats to Lorenz and the team). The announcement could also be viewed as the starter gun on a new race as additional entrants now rush to get their own studies started.
It will be interesting to see how this race plays out.
 All of the material on this website is licensed under a
All of the material on this website is licensed under a
Creative Commons Attribution 4.0 International License
You can do whatever you like with it!
Addendum: 19th July, 2021 – BlueRock Therapeutics announced that the U.S. Food and Drug Administration (FDA) has granted “Fast Track designation” for DA01 for advanced Parkinson’s (Click here to read the press release).
Fast Track designation is designed to expedite the regulatory review process, providing more frequent meetings and interactions with FDA. It involves a rolling review pathway, and can make the treatment eligible for “Accelerated Approval” (if relevant criteria are met).
In a nut shell: it is good news.
FULL DISCLOSURE – The author of this blog is employed by Cure Parkinson’s which is funding the ongoing Transeuro cell transplantation trial. Cure Parkinson’s and associated parties have not requested that this material be produced. The author is providing it for educational purposes.
In addition, some of the companies mentioned in this post are publicly traded companies. That said, the material presented on this page should under no circumstances be considered financial advice. Any actions taken by the reader based on reading this material is the sole responsibility of the reader. None of the companies mentioned have requested that this material be produced, nor has the author had any contact with the companies.
It is also important for all readers of this post to appreciate that cell transplantation for Parkinson’s disease is still experimental. Anyone declaring otherwise (or trying to sell a procedure based on this approach) should not be trusted. While I appreciate the desperate desire of the Parkinson’s community to treat the disease ‘by any means possible’, bad or poor outcomes at the clinical trial stage for this technology could have serious consequences for the individuals receiving the procedure and negative ramifications for all future research in the stem cell transplantation area.
The banner for today’s post was sourced from BlueRock














