|
# # # # The first post at the start of each year on the SoPD website tries to provide an overview of where things are in the search for ‘disease modifying’ therapies for Parkinson’s. It is an exercise in managing expectations as well as discussing what research events are scheduled for the next year so that we can keep an eye out for them. I will also note aspects of ongoing research where I will be hoping to see an update on progress. Obviously, where 2022 will actually end is unpredictable, but an outline of what is coming over the next 12 months will hopefully provide the community with a useful resource. While there is a great deal of interesting research exploring the causes of the condition, the genetics and biology of the condition, novel symptomatic therapies, and other aspects of Parkinson’s, the primary focus in this post is on the clinical trial research seeking to slow, stop or reverse the condition. In this post, we will hopefully give readers a taste of what the landscape looks like for clinical research focused on disease modification for Parkinson’s. # # # # |
 Source: CT
Source: CT
“If you have men who will only come if they know there is a good road, I don’t want them. I want men who will come if there is no road at all.”
―
The Scottish physician Dr David Livingstone – missionary and explorer – led an interesting life.
Most of us only know of him for his fabled adventures in Africa. But they are made more remarkable given his extremely humble beginnings.
Born into poverty, Livingstone started his working life at 10 years of age in a cotton factory, where he worked from 6am till 8pm everyday. He somehow managed to get some schooling around those work shifts, and his impoverished family saved enough money so that he could attend Anderson’s University (Glasgow) when he was 23.
 Cotton factory (Source)
Cotton factory (Source)
How he got from the cotton factory to becoming the first European to cross the width of southern Africa (as well as ‘discovering’ the Mosi-oa-Tunya waterfalls – aka Victoria Falls), was one of the great rag-to-riches stories of Victorian times and making him something of a celebrity of the age.
 Mosi-oa-Tunya waterfalls. Source: Cblacp
Mosi-oa-Tunya waterfalls. Source: Cblacp
But his mapping out of central Africa was his greatest legacy.
As a biographer wrote “Through him, the centre of Africa ceased to be a dark, unknown space on the map and became a real place, full of interesting human beings [and] wonderful wildlife. . . .” (Source)
It has to be acknowledged, however, that Livingstone was not able to explore the entirety of the Zambezi River system himself so he would often ask the local people for information, and he would then incorporate their contributions into his maps.
 Livingstone’s travels (Source)
Livingstone’s travels (Source)
“We travel in the company of men who are well acquainted with parts of the country by personal observation… They soon see that we are interested in the courses of rivers, names of hills, tribes…and make enquiries among the villagers to whom we come. Drawings are made on the ground and parts pointed out that bearings may be taken and comparisons drawn from the views of different individuals. We thus gain a general idea of the whole country” (Source)
It makes one appreciate that maps are collaborative efforts, incorporating the efforts of lots of different parties. And it is only by going through the process of mapping something out that we start to understand it, know our place in it, observe the limitations to our knowledge, and perhaps find something of what we are looking for.
At the start of each year, the SoPD publishes a horizon scanning post where we take a Livingstone-like approach towards mapping out the landscape of clinical research focused on disease modification for Parkinson’s, and what follows is the 2022 version.
To be clear, this post is NOT intended to be an exercise in the reading of tea leaves (no predictions will be made here). Nor is this a definitive or exhaustive guide of what the next year holds for disease modification research (if you see anything important that I have missed – please contact me or leave a comment below).
Readers should be aware that there is still a long way to go before any of these potential therapies will be made available in the clinical setting. Of the Phase III clinical trials, exenatide is the closest experimental therapy to any kind of clinical approval and that study will not be finished before 2024. And it should certainty not be assumed that it or any of the other treatments being discussed are going to be silver bullets or magical elixirs that are going to “cure” the condition on their own.
As I have often discussed on this website, a “curative therapy” for Parkinson’s is going to require three core components:
- A disease halting mechanism
- A neuroprotective agent
- Some form of restorative therapy
Now, the bad news is (as far as I am aware) there is no single treatment currently available (or being tested) that can do all three of these things. By this I mean that there is no disease halting mechanism therapy that can also replace lost brain cells. Nor is there a restorative therapy that stop the progression of the condition.
That last paragraph can obviously be read as bad news, but it shouldn’t.
Let me explain:
A curative therapy for Parkinson’s is going to need to be personalised to each individual, with varying levels of each of the three component listed above. It will be a multi-modal approach, designed to best fit each individual’s needs.
 Making things personal. Source: Flickr
Making things personal. Source: Flickr
By this I mean, there is a great deal of heterogeneity (or variability) between individuals with regards to their Parkinson’s symptoms and the amount of time that they have had the condition. No two cases of Parkinson’s are the same. Some folks are more tremor dominant, while others do not experience tremor at all. Likewise, some individuals have only just been diagnosed, while others have lived with the condition for many years.
As a result, the treatment needs of each individual will be different, and thus what we will require is differing amounts of each component for each individual. By this I mean, that someone who has only just been diagnosed may only need the disease halting mechanism component, while someone who has had the condition for many years will need different amounts of all three components (depending on their situation).
Now the good news is that there is considerable clinical research currently being conducted on each of these three components. And we will now explore what research is happening in each of these components and discuss what is scheduled for 2022.
|
IMPORTANT NOTE: Before we start, this website is the personal blog of the deputy director of research at Cure Parkinson’s. The Trust is a UK registered research charity which is an international supporter of many of the clinical trials mentioned in this post. To avoid any bias and for the purposes of full disclosure, where appropriate I will note the Trust’s involvement. In addition, I would like to thank Parkinson’s research advocates Sue Buff and Kevin McFarthing for the efforts they put into maintaining their wonderful databases of Parkinson’s clinical trials (Sue maintains the PDTrialTracker website, while Kevin keeps the “Hope list“). This post would not be possible without their amazing resources. # # # # |
This post is a rather long one (in future years I might break it down into separate posts), so to save the reader’s sanity, a contents index is being provided here. Hopefully when you click on the section that interests you in this index, you will jump to that section. To come back to the index, simply hit the ‘return’ button on your browser.
THE INDEX
COMPONENT #1: A disease halting mechanism
- The direct approach
- The direct approach: Alpha synuclein
- Passive immunotherapy approaches for alpha synuclein
- Active (vaccine) immunotherapy approaches for alpha synuclein
- Small molecule approaches targeting alpha synuclein
- The direct approach: LRRK2
- The direct approach: GBA
- The direct approach: Mitochondria
- The direct approach: Additional
- The indirect approach
- The indirect approach: Autophagy
- The indirect approach: Inflammation
- The indirect approach: Gastrointestinal system
- The indirect approach: Iron chelation
COMPONENT #2: A neuroprotective agent
- GLP-1 agonists
- Neurotrophic factors
- Glial cell-derived neurotrophic factor (or GDNF)
- Cerebral dopamine neurotrophic factor (or CDNF)
- Other neurotrophic factors
- Neuroprotective approach: Additional
COMPONENT #3: Some form of restorative therapy
I hope this helps with navigating this extremely long post (you have been warned: it’s a doozie!).
Let’s now consider the first component of any curative therapy for Parkinson’s:
COMPONENT #1. A disease halting mechanism
Parkinson’s is a progressive neurodegenerative condition. Thus, the first and most critical component of any ‘curative therapy’ for Parkinson’s involves a treatment that will slow down or halt the progression of the condition.
This can be done either directly or indirectly.
The direct approach
The direct approach involves treatments that specifically target the underlying biology of the condition.
A direct approach in halting Parkinson’s, however, requires a fundamental understanding of how the condition is actually progressing. And if we are honest, we are not there yet – we still do not have a solid grasp of how Parkinson’s progresses over time. In addition, this may vary between individuals. It is gradually being agreed that rather than being a single ‘disease’, Parkinson’s may actually be a ‘syndrome’ – that is, a collection of conditions that share similar symptoms.
We do, however, have some solid theories as to what is happening, and there are numerous clinical trials focused on attempts at “direct approaches” to halting Parkinson’s.
For example, there is a protein called alpha synuclein which is known to build up in neurons in many cases of Parkinson’s. Pathologists consider this one of the characteristic features of the Parkinsonian brain.
The direct approach: Alpha synuclein
When it is made, alpha synuclein protein looks like this:
 Alpha synuclein. Source: Wikipedia
Alpha synuclein. Source: Wikipedia
For some reason in Parkinson’s, the protein begins to cluster and aggregate. This build up of alpha synuclein protein is associated with the appearance of structures called Lewy bodies in the brains of people with Parkinson’s.
 A lewy body (brown with a black arrow) inside a cell. Source: Cure Dementia
A lewy body (brown with a black arrow) inside a cell. Source: Cure Dementia
It is believed that alpha synuclein might be passed from cell to cell and ‘seeding’ the condition in each cell as it goes. Some researchers propose that this ‘pass-the-parcel’ mechanism may underlie the slow progressive nature of Parkinson’s. Researchers have proposed that targeting alpha synuclein as it is being passed between cells could represent a means of slowing/halting the progression of Parkinson’s.
 The passing of alpha synuclein between brain cells. Source: Nature
The passing of alpha synuclein between brain cells. Source: Nature
One of the direct approaches being employed against alpha synuclein is a method called immunotherapy.
Immunotherapy involves boosting the body’s immune system to target specific toxic agents in the body. In the case of Parkinson’s, this approach is primarily being focused on different forms of alpha synuclein.

Antibodies. Source: Astrazeneca
The immunotherapy approach uses antibodies, which are Y-shaped proteins that act like alert flags for the immune system. Once enough antibodies bind to a particular object, the immune system will dispose of it. Antibodies target very specific structures, while ignoring everything else.
In Parkinson’s, the immunotherapy approaches are primarily involving antibodies that target the alpha synuclein protein. By tagging the alpha synuclein as it is being passed from one cell to another, and allowing the immune system to remove it, researchers hope to slow down the progression of Parkinson’s.
Immunotherapy can be conducted in two ways:
- The body’s immune system can be encouraged to develop its own antibodies that target the toxic form of alpha synuclein (using active immunisation in the form of a vaccine); or
- Researchers can design antibodies themselves that specifically target the toxic form of alpha synuclein (while leaving the normal version of the protein alone), and then inject those antibodies into the body (passive immunisation)
 Immunotherapy. Source: Acimmune
Immunotherapy. Source: Acimmune
Passive immunotherapy approaches for alpha synuclein:
There are now numerous biotech firms testing passive immunotherapy approaches in the clinic for Parkinson’s, but in reality there have been two main study programs in the passive immunotherapy for Parkinson’s that everyone has been keeping their eyes on (simply because of their advanced nature in the clinical trial process).
The first is the PASADENA study.
In April 2020, the results of the PASADENA study were announced. This study was a Phase II clinical trial of an alpha synuclein targeting immunotherapy (called Prasinezumab – formerly called RO7046015 & PRX002) that was conducted by the pharmaceutical company Roche and biotech firm Prothena Biosciences (Click here to read a SoPD post about this).
The companies announced that the trial had not met its primary endpoint (a predetermined measure of efficacy), but that prasinezumab “showed signals of efficacy” , and importantly: “These signals were observed on multiple prespecified secondary and exploratory clinical endpoints“ (Source).
In September, the companies clarified this statement in another press release (Source). When the researchers looked at just the motor scores of the participants (UPDRS Part III), there was evidence of a slower progression in the participants treated with prasinezumab than those treated with placebo (remember, these individuals were all blind to their treatment):
 Source: Prothena
Source: Prothena
Roche and Prothena have concluded that their “findings support the potential of prasinezumab to slow underlying disease pathophysiology and clinical decline in patients with PD. Further investigations are warranted” (Source) and the two companies are continuing to follow up with participants in the current Phase II study.
The baseline data for this study has been published (Click here to read more about this), but additional post hoc analysis data was presented at the 15th International Conference on Alzheimer’s and Parkinson’s Diseases (March 9–14th) which suggested that the “slight slowing of motor decline by prasinezumab was more evident in subgroups of patients whose disease progressed faster, such as those on MAO-B inhibitors (middle) and those with a more aggressive form of PD” (Source).
 Source: Alzforum
Source: Alzforum
It is very important to remember that these observations are based on post hoc analysis (that is, after-the-fact re-analysis of the trial data) and they should not be considered as evidence of efficacy. But it would be encouraging if additional immunotherapy trials show signals that also warrant further investigation.
Speaking of which… in May 2021, Roche and Prothena began a Phase IIb trial – called the PADOVA study – of prasinezumab in patients with early Parkinson’s. The study is enrolling 575 people (who are on stable dopamine replacement medication) who will be randomised to monthly treatment of either prasinezumab or placebo for 18 months. The primary endpoint of this study is MDS-UPDRS Part III, and the results will hopefully be available in 2024 (Click here to read more about this study)
Sounds very positive, right?
Well, the second main immunotherapy study for alpha synuclein in Parkinson’s was the SPARK study which was conducted by the Pharmaceutical company Biogen.
 And the results of that program were not as encouraging.
And the results of that program were not as encouraging.
The results of Phase I testing of Biogen’s alpha synuclein targeting immunotherapy treatment – called Cinpanemab (also known as BIIB054) – had demonstrated that the treatment was safe and well tolerated (Click here to read a SoPD post about the Phase I Biogen study results), and so the company had plowed ahead with the carefully designed Phase II SPARK trial.
This was also a 2-year Phase II clinical trial that was testing Cinpanemab in 300+ people with Parkinson’s. In the first year of the study, participants in the study were randomly assigned to monthly infusions of 3 different doses of Cinpanemab (250mg, 1250mg, or 3500mg) or a placebo treated group (Click here to read more about this study). At the start of year two, members of the placebo group were switched to receive the Cinpanemab treatment as well.
 Unfortunately, in February 2021, Biogen announced to their investors – in a single sentence buried deep in their annual results (PDF) – that the company had halted development of cinpanemab after the SPARK study missed its primary and secondary endpoints.
Unfortunately, in February 2021, Biogen announced to their investors – in a single sentence buried deep in their annual results (PDF) – that the company had halted development of cinpanemab after the SPARK study missed its primary and secondary endpoints.
The Parkinson’s communities are still awaiting the publication of the final results of this study.
In addition to the Pasadena and SPARK studies, there are a large number of other biotech companies developing immunotherapy programs for Parkinson’s, including:
- Astrazeneca‘s immunotherapy treatment called MEDI1341 (being developed with Takeda Pharmaceutical) completed Phase I safety testing in healthy volunteers in early 2021 (Click here to read more about that study). In addition, in June 2020, Astrazeneca registered a second Phase I study assessing multiple ascending doses of MEDI1341 in people with Parkinson’s. That new study is scheduled to complete in July 2022 (Click here to read more about this trial). The company has published preclinical research on this agent (Click here to read more about that).
- Lundbeck‘s immunotherapy treatment called Lu AF82422 (which is being developed in collaboration with Genmab) was in Phase I safety testing in both healthy volunteers and people with Parkinson’s during 2020 and it was completed in December 2020 (Click here to read more about this). In November 2021, the company initiated the AMULET study – a Phase II clinical trial of Lu AF82422 in individuals with multiple system atrophy (MSA, which is a condition very similar to Parkinson’s). The study will involve 60 patients (40 in the Lu AF82422 group and 20 in the placebo group) who will be treated for 72 weeks (Click here to read the press release and click here to read more about this study). It will be interesting to learn if 2022 if they will conduct a PD trial in parallel.
- In March 2020, the pharmaceutical company AbbVie started a multicenter, placebo-controlled Phase I study of their immunotherapy treatment called BAN0805/ABBV-0805 (Click here to read more about this – this immunotherapy approach is being developed in collaboration with BioArctic Neuroscience). In June 2020, however, the Phase I study was withdrawn and BioArctic announced that a detailed plan to accelerate ABBV-0805 into a Phase II Proof of Concept study in Parkinson’s is now being prepared by AbbVie (Source). ABBV-0805 differs from most of the antibodies under clinical investigation which are of the IgG1 subclass, while ABBV-0805 is a stabilized IgG4 molecule (this has the benefit of lacking complement binding function). The companies have published preclinical data on ABBV-0805 (Click here to read that research), and it is stated that “ABBV-0805 has been progressed into clinical development“, but there has been no news regarding further clinical development. Hopefully we will learn more in 2022. It is interesting to note that these two companies have two additional alpha synuclein-targeting immunotherapy treatments called PD1601 & PD1602 also in development.
- Very recently the pharma company Novartis has signed a licensing deal with the Belgium biotech giant UCB to develop alpha synuclein targeting therapies. As part of that agreement, the company has the option to develop an immunotherapy called UCB7853 which is currently in Phase I testing (Click here to read more about this study). The two companies will split the cost of development and – if approved – the drug will be sold by UCB in Europe and Novartis in the rest of the world (Click here to read a recent SoPD post on this topic).
- Another member of the big pharma community that has recently entered the immunotherapy for Parkinson’s space is the French drug giant Sanofi with the signing of a licensing deal with the South Korean biotech firm ABL Bio. The deal gives Sanofi the right to develop and commercialise ABL301, which is an anti-alpha synuclein antibody. This immunotherapy has a unique feature as it also carries an additional Grabody-B component to maximize blood-brain barrier penetration (which has been an issue for previous immunotherapies). ABL Bio will now conduct Phase I testing of ABL301, and we will hopefully learn more about this in 2022 (click here to read the press release about this).
|
Preclinical developments in passive immunotherapies to look out for in 2022: In addition to the companies with clinical programs there are other biotech firms developing antibody-based therapies targeting alpha synuclein for Parkinson’s. These include:
There are also several companies developing gene therapy-based immunotherapy approaches focused on alpha synuclein, including:
|
Now you may recall that we mentioned two types of immunotherapy above – passive and active (‘passive’ requiring regular injections of antibodies, while ‘active’ enables the immune system to produce the antibodies, requiring less treatments). The clinical trials we have discussed above is passive immunotherapy.
In addition to these passive immunotherapy treatments, there are also two biotech companies that are clinically testing active immunotherapy treatment in Parkinson’s. These are vaccines for Parkinson’s, which targets the toxic form of alpha synuclein.
Active (vaccine) immunotherapy approaches for alpha synuclein:
The company with the most advanced vaccine program is called AFFiRiS.

They have been clinically testing a vaccine treatment called ‘PD01A’ and in July 2020, the results of their Phase I studies were published in the journal Lancet Neurology. (Click here to read more about this).
While it is important to remember that this trial was an ‘open label’ study (meaning that all of the participants knew what they were being treated with and a placebo response could have been at play), the results were rather interesting. Firstly that the treatment is safe and well tolerated in the participants, and the vaccine caused the immune system to start producing alpha synuclein targeting antibodies. In addition, by 26 weeks into the study, the researchers observed a 51% reduction in cerebrospinal fluid levels of aggregated alpha synuclein.
Regarding some basic assessments of disease progression, the researchers wrote in their report that: “DAT-SPECT examinations did not show statistically significant changes up to 91 weeks in study 1. MDS-UPDRS part 3 scores were generally stable across the studies”.
This sentence suggests that the researcher did not see any brain imaging or clinical evidence of disease progression. But again, this was an open label study, and a larger, double blinded evaluation of the PD01A treatment is now required.
In January 2020, AFFiRiS announced that based on feedback from the US FDA, they could proceed with preparations for a Phase II clinical trial. They planned to initiation that study in the US and Europe in the second half of 2020 (click here to read more about this), but COVID-19 appears to have delayed those plans. And then in 2021, it was announced that the Switzerland-based biotech company AC Immune was acquiring the Parkinson’s-associated immunotherapy assets off AFFiRiS and taking them forward in clinical development (Click here to read a SoPD post on this topic).
AC Immune announced that they would be immediately launching “clinical development of ACI-7104 [formerly PD01A ], the optimized formulation of PD01, into an adaptive, biomarker-based Phase 2 study”. We look forward to learning more about this new study in 2022.
A second company developing a vaccine against alpha synuclein is Vaxxinity (formerly known as “United Neuroscience”).
This biotech company is focused on developing a novel class of vaccines that are fully synthetic (they call them ‘endobody vaccines‘) and can train the body to treat/prevent neurological condtions. They are currently conducting a Phase I safety/tolerability trial of UB-312 in healthy volunteers and in participants with Parkinson’s. Very recently, the company announced that they had finished Part 1 of this study (involving the healthy volunteers) and they had dosed the first participant in Part 2 of the study, involving individuals with Parkinson’s (Click here to read the press release). The study is scheduled to complete in December 2022 (Click here to read more about this trial).
|
Preclinical developments in active immunotherapies to look out for in 2022: As with the passive immunotherapies, there are also biotech companies with active immunotherapies (vaccines) in preclinical development.
|
One of the acknowledged limitation of the immunotherapy approaches, is the low amount of antibody actually accessing the brain (though Denali Therapeutics & Biogen are collaboratively developing technology to improve this situation – click here to read a previous SoPD post about this).
In the most of the immunotherapy trials to date, only 1-3% of the treatment in the blood is actually getting into the brain. This is due to a protective membrane surrounding our brains, called the blood brain barrier, which limits entry of most drugs/proteins.
These limited amounts of immunotherapy treatment still allowed for the clearance of the targeted protein (beta amyloid in the case of the Alzheimer’s trials) so it can be assumed that it should also be enough to be able to reduce levels of extracellular alpha synuclein in the Parkinson’s immunotherapy clinical trials.
But these immunotherapy trials will also have limited ability to affect alpha synuclein within cells (remember, they are tagging and grabbing the protein as it is being passed between cells). This situation has led a growing number of biotech companies to develop small molecules that can actually enter and target alpha synuclein inside of cells.
Small molecule approaches targeting alpha synuclein:
After a long period of watching immunotherapy approaches for alpha synuclein being tested in the clinic, we are now seeing an increasing number of small molecule inhibitors of synuclein aggregation entering the fray. And this is an extremely encouraging development as this class of molecules will allow us to truly test the hypothesis that alpha synuclein is involved in the pathology of Parkinson’s.
The most advanced of these molecules (in terms of their clinical development) is a repurposed agent – the tricyclic antidepressant nortriptyline. This is currently being clinically evaluated in the Antidepressants Trial in Parkinson’s Disease (or ADepT-PD) study.
 This placebo-controlled study is a Phase III clinical trial assessing the drugs nortriptyline and escitalopram on depression in Parkinson’s. It involves 408 participants and is currently ongoing at 30 research sites across the UK (Click here to learn more about this study).
This placebo-controlled study is a Phase III clinical trial assessing the drugs nortriptyline and escitalopram on depression in Parkinson’s. It involves 408 participants and is currently ongoing at 30 research sites across the UK (Click here to learn more about this study).
Within the ADepT-PD study, Cure Parkinson’s is supporting a sub-study which will be investigating the disease modifying potential of nortriptyline (Click here to read more about this sub-study).
This study was initiated on research indicating that people newly diagnosed with Parkinson’s who are also on tricyclic antidepressants have a longer period of time before requiring dopamine-based treatment (eg. L-dopa):
 PMID: 22555881
PMID: 22555881
Supportive subsequent preclinical data demonstrating neuroprotective in models of PD was encouraging enough to provide a further case for supporting a clinical evaluation of nortriptyline.
This ADepT-PD trial is currently recruiting and involves 12 months of treatment – Click here if you are interested in learning more about potentially taking part in this study and click here to read a recent SoPD post discussing this topic.
Another small molecule inhibitor of alpha synuclein program that is advancing through the clinical trial process is being led by the biotech firm Annovis (formerly QR Pharma). They are developing an agent called Buntanetap (previously known as ANVS-401 or Posiphen) for the treatment of Parkinson’s and Alzheimer’s.
 Buntanetap functions by binding to a region of RNA that is shared by alpha synuclein RNA (and also the Alzheimer’s-associated beta amyloid RNA), and this action inhibits the translation of the RNA into the alpha synuclein protein.
Buntanetap functions by binding to a region of RNA that is shared by alpha synuclein RNA (and also the Alzheimer’s-associated beta amyloid RNA), and this action inhibits the translation of the RNA into the alpha synuclein protein.
 Source: SEC
Source: SEC
In October 2021, Annovis announced the results of a small Phase IIa trial evaluating various doses of buntanetap over 1 month of administration. The study involved 14 people with PD and 14 with Alzheimer’s, and it was mainly exploring safety/tolerability as well as some exploratory functional and cognitive endpoints (Click here to read more about this study). An extension of the PD arm of the study included an additional 40 individuals with Parkinson’s, taking the total to 54 PD patients.
The results indicated a slight placebo response in terms of total UPDRS scores:
 Source: Annovis
Source: Annovis
But when the investigators zeroed in on Part III of the UPDRS (the motor symptoms), they reported an improvement over the 1 month study (compared to the placebo treated group):
 Source: Annovis
Source: Annovis
Annovis has recently announced that they have received guidance from the US FDA on specific endpoints, entry criteria, & further study parameters for two Phase III clinical trials of buntanetap that “would support a broad indication for both early & late Parkinson’s” (Source). So I am assuming that we may see at least one Phase III trial for buntanetap starting in 2022.
A third small molecule inhibitor of alpha synuclein that is being clinically tested is Anle138b, which is being developed by the biotech firm MODAG.
 Phase I clinical testing of this drug was initiated in 2019 (Click here to read more about this), and in August 2020 MODAG announced that they had completed the study (Click here to read the press release). Anle138b is currently being targeted by the company toward Multiple System Atrophy – a neurodegenerative condition similar to Parkinson’s – but MODAG is also very keen to test the molecule in Parkinson’s and this was made apparent as they initiated a Phase Ib clinical trial of Anle138b in individuals with Parkinson´s in December 2020 (Click here to read the press release and click here to read more about this trial). This second study is scheduled to finish in June 2022.
Phase I clinical testing of this drug was initiated in 2019 (Click here to read more about this), and in August 2020 MODAG announced that they had completed the study (Click here to read the press release). Anle138b is currently being targeted by the company toward Multiple System Atrophy – a neurodegenerative condition similar to Parkinson’s – but MODAG is also very keen to test the molecule in Parkinson’s and this was made apparent as they initiated a Phase Ib clinical trial of Anle138b in individuals with Parkinson´s in December 2020 (Click here to read the press release and click here to read more about this trial). This second study is scheduled to finish in June 2022.
In October 2021, the pharmaceuticals company Teva announced a strategic collaboration with MODAG for the exclusive worldwide licensing and development of Anle138b and a related compound, sery433 (Click here to read more about this).
Sery433 appears to be a prodrug for Anle138b – it will be interesting to learn more about this molecule in 2022, and to hear news about future developments of Anle138b for PD.
Another experimental small molecule targeting alpha synuclein that is in clinical testing at the moment is ENT-01, which is being developed by the biotech company Enterin Inc.
ENT-01 is a synthetic version of squalamine, a molecule originally discovered in the liver and gall bladder of the dogfish shark.
Squalamine has a wide range of antimicrobial activities, but in 2017 researchers discovered that it is also a potent inhibitor of alpha synuclein protein aggregation:

PMID: 28096355
Enterin Inc. was foundered and between 2017-2018, the company conducted the RASMET study, which was a Phase I safety clinical trial of ENT-01 (Click here for the details about this trial and click here to read a SoPD post on this topic). The issue with ENT-01 compared to other molecules targeting alpha synuclein is that it does not cross the blood brain barrier. Thus, Enterin are focusing their clinical trial on Parkinson’s-associated constipation – can this drug reduce alpha synuclein aggregation in the gut and alleviate complaints like constipation.
The results of the Phase I RASMET study have been published (Click here to read them and click here to read the press release), and the company has very recently announced the results of their Phase IIb ‘KARMET’ clinical study of ENT-01 (Click here to read more about this study).
KARMET was a randomized, placebo-controlled, double-blind study of ENT-01 involving 150 individuals with Parkinson’s. Following a 2-week baseline period, participants were stratified to high dose or low dose depending on baseline constipation severity & randomized to receive ENT-01 or placebo. They were treated and monitored for a 25-day period, then all placed on placebo for 2 weeks before going through a 4-week wash-out. ENT-01 was again found to be safe & well tolerated, with common adverse events being primarily gastrointestinal in nature.
The primary endpoint in the study – change in complete spontaneous bowel movement from baseline to the end of the 3-week treatment period – was met. The researchers observed that bowel movement was significantly better in the ENT-01 treatment group compared to placebo (p=0.0001). It is interesting to note that there was some maintenance of this effect in the washout phase:
 Source: Enterin
Source: Enterin
In addition, all of the bowel-related secondary endpoints improved in the ENT-01 treatment group. Interestingly, there was a reduction in levels of psychosis (as measured by SAPS-PD) during 3 week study period, & effect persisted out to 6 weeks post termination of treatment (small numbers in this result, but trend is present)
 Source: Enterin
Source: Enterin
Motor scores (as determined by UPDRS III) were measured, but this was mainly done for safety reasons (the 3 week study was too short for any meaningful efficacy measures). The results indicated that there was no worsening of motor symptoms during the study for either treatment group. Enterin is yet to announce what the next steps are in terms of clinical testing of ENT-01 for Parkinson’s, and they may now be seeking guidance from the FDA regarding next steps.
Enterin has also been assessing ENT-01 in Parkinson’s Disease Dementia in a Phase I open label study (Source). This study was scheduled to complete in mid-2021, so we will hopefully also learn the results of this study in 2022.
In addition to ENT-01 which does not cross the blood brain barrier, Enterin has a strong patent position around another molecule called Trodusquemine which does get into the brain. It is very similar to ENT-01 (also known as squalamine):
 Source: Modernsteroid
Source: Modernsteroid
The assumption here at SoPD HQ is that the company is conducting preclinical evaluations of trodusquemine (or a derivative of it) with the goal of taking it forward for Phase I clinical testing. We hope to learn more about this in 2022.
One of the more novel alpha synuclein-targeting approaches for Parkinson’s is being clinically tested by the biotech company Yumanity.

This company is developing Stearoyl CoA desaturase (SCD) inhibitors – these are a class of drugs that have been reported in preclinical research to reduce alpha synuclein-associated toxicity (Click here to read a SoPD post on this topic).
In October 2019, Yumanity initiated Phase I clinical testing their first drug, YTX-7739, in 48 healthy individuals (Click here to read the press release and click here to read more about that trial), and then another Phase I trial study exploring multiple doses in people with mild-to-moderate Parkinson’s (Click here to read more about this trial).
In November 2021, the results of these studies were announced, and the investigators reported that the drug was generally well tolerated with no serious adverse events, plus favorable pharmacokinetic/pharmacodynamic (PK/PD) profiles. In addition, there was evidence of target engagement, with markers of SCD inhibition reducing by approximately 20%-40% (Click here to read more about this).
The company are now planning a Phase II randomized clinical trial in Parkinson’s, which they expect to start in 2022.
Another small molecule inhibitor of alpha synuclein that is advancing through clinical testing is UCB0599 (previously known as NPT200-11). In December 2021, the pharmaceutical company Novartis announced that they were forming a global co-development and co-commercialization agreement with the pharmaceutical company UCB who have been developing UCB0599 (Click here to read the press release).
 This was an interesting move for Novartis, who have had little interest in PD up to this point (Click here to read an SoPD post on this collaboration). UCB0599 was originally discovered by a biotech company called Neuropore Therapies.
This was an interesting move for Novartis, who have had little interest in PD up to this point (Click here to read an SoPD post on this collaboration). UCB0599 was originally discovered by a biotech company called Neuropore Therapies.
After licensing UCB0599 from Neuropore Therapies, UCB initiated the Phase IIa “ORCHESTRA” trial for the agent in December 2020. This is a double-blind, placebo-controlled, randomized, 18-month study to assess the safety and tolerability of UCB0599 in 450 people with Parkinson’s.
The study will be looking for any evidence that UCB0599 is superior to placebo in terms of slowing disease progression over 12 and 18 months (the primary outcome of the trial is MDS-UPDRS Parts I-III sum score). The study is now recruiting individuals with early stage Parkinson’s at research sites in the U.S., Canada, and the Netherlands and it is scheduled to finish in July 2024 (Click here to read more about this trial).
And finally, a topic of great interest to many readers of the SoPD has been the sweetner Mannitol, which – after some interesting preclinical results – was crowd sourced into a patient-led online study by a group called Clinicrowd (Click here to read an interesting write up on this and related efforts).
The results of that online study have been published (Click here to read a previous SoPD post about this topic), and have stimulated a Phase I dose escalation clinical trial in Israel (Click here to read more about that trial). That trial finished in December 2020 and the results were recently published (Click here to read the report). The study was small (only 14 individuals on mannitol and 8 on a control treatment) and it was primarily focused on the safety and tolerability of mannitol treatment over a 36 week period.
The results indicate that “gastrointestinal symptoms limited dose escalation in 6/14 participants on mannitol” and there was no recovery of sense of smell in any participants (this was an observation from the earlier online study). The study was not statistically powered to demonstrate efficacy, but the researchers noted that no difference in clinical symptoms was observed between the mannitol and control groups.
No measure of mannitol in the brain was made, so it is still unclear as to what degree the molecule accesses the central nervous system after oral administration. It is also unclear whether a larger clinical trial is planned. Perhaps we will learn more in 2022.
As you can see there are a lot of alpha synuclein-focused clinical trial programs.
|
Preclinical developments in alpha synuclein-targeted small molecules to watch out for in 2022: Novel molecules:
Gene therapy approaches:
PROTAC(-like) compounds: Proteolysis targeting chimera (or PROTAC) technology is a system of targeting and degrading intracellular proteins. Companies exploring alpha synuclein-targeted PROTACs (or PROTAC-like approaches) include:
|
Not all of the direct approaches for slowing the progression of Parkinson’s involve targeting alpha synuclein protein. In fact, there are some cases of Parkinson’s that do not appear to involve any accumulation of alpha synuclein at all. For example, in many cases of LRRK2-associated Parkinson’s, postmortem analysis has indicated that there is very limited alpha synuclein.
The absence of alpha synuclein pathology in these LRRK2-associated Parkinson’s cases, has led to a new class of therapeutics attempting to target LRRK2 itself.
The direct approach: LRRK2
Leucine-rich repeat kinase 2 (or LRRK2 – pronounced ‘lark 2’) is a Parkinson’s-associated protein that can become hyperactive in some people with Parkinson’s.
Some individuals carry genetic variations in the region of DNA that provides the instructions of making LRRK2 protein, while other individuals with idiopathic PD have elevated levels of LRRK2 protein for reasons that are yet to be determined. This over-active form of the protein is believed to be associated with the neurodegeneration.
To try and inhibit the over-active form of this protein in the carefully balanced environment of cells, researchers have been developing LRRK2 inhibitors. The hope is that by inhibiting LRRK2, function in the cell will be able to return to normal (or more manageable levels) which will make cells healthier. By doing this we may be able to slow down/halt the cell death and stablise the course of Parkinson’s.
Leading the pack in the race to develop LRRK2 inhibitors is a biotech firm called Denali Therapeutics.
Set up by a group of ex-Genentech scientists, Denali has been clinically testing two LRRK2 inhibitors: DNL-151 and DNL-201. In 2020, the company announced that they have finished Phase I testing of these drugs and they signed an agreement with the pharmaceutical company Biogen to co-develop and co-commercialise DNL151 (also being called BIIB122) as the lead LRRK2 inhibitor.
In 2021, Denali published their Phase I results of the clinical testing of DNL151 (Click here to read a previous SoPD post about this). Collectively, all of the Phase I studies involved 184 healthy volunteers (145 administered with DNL151 & 39 with placebo) and 36 people with Parkinson’s (26 administered with DNL151, 10 with placebo), so a lot of data was collected regarding the pharmacokinetics and pharmacodynamics of DNL151.
The results indicate that DNL151 was safe and generally well tolerated. No serious adverse events were observed and the majority of treatment-emergent adverse events reported were mild in nature and resolved after termination of treatment. Importantly, there were no clinically meaningful changes in pulmonary or renal function in either study. Overall, a positive outcome considering that this is a new drug class in humans.
The researchers in these studies also investigated various biomarkers of LRRK2 activity and measures of target engagement. For example, levels of the phosphorylated form of LRRK2 – known as pS935 LRRK2 – were measured in blood samples, and found to be less than half that observed in the placebo treated group across all of the three doses tested in the Parkinson’s patients (80 mg, 130 mg, and 300 mg given once daily for 28 days):
 QD means ‘once a day’. Source: Denali
QD means ‘once a day’. Source: Denali
All of the data presented demonstrates that DNL151/BIIB122 is inhibiting LRRK2 and it is now ready for some evaluations of efficacy.
In July of 2020, the US FDA cleared an Investigational New Drug (IND) application for DNL151/BIIB122 enabling an expansion of Denali’s clinical trial program. Biogen and Denali have recently announced their plans for future development of DNL151/BIIB122 (Source).
There will be two studies:
- The first trial will be called the “LIGHTHOUSE study” and it will be a global Phase III clinical trial. The companies are hoping to recruit 400 people with Parkinson’s who carry a variation in their LRRK2 gene. These individuals will be treated with either BIIB122 or placebo for at least 96 weeks, and the companies have suggested that the study is “designed to potentially support registration” (Source).
- The second trial will be called the “LUMA Study” and it will be a large Phase IIb clinical trial that will enroll 640 individuals with Parkinson’s. Importantly in this study, the participants will NOT have any genetic variant in their LRRK2 gene. This study will also be shorter than the LIGHTHOUSE study as the treatment period (of BIIB122 or placebo) will only be 48 weeks.
In both of the studies, the clinical symptoms of the participants will be assessed over time and the success of the study will be determined by whether there is a decrease in the rate of progression in the individuals treated with BIIB122. Enrollment for these trials is expected to commence in 2022. Denali has set up a website (EngageParkinson’s) for anyone seeking to learn more.
In 2022, we will also be looking for updates from Denali therapeutics regarding their collaboration with gene therapy company, SIRION Biotech.
 Denali signed a collaboration in early 2019 to explore vectorising their LRRK2 inhibitors, suggesting that while the initial proof-of-concept test for LRRK2 inhibition is focused on oral treatment, the long-term future may be more focused on gene therapy approaches (Source).
Denali signed a collaboration in early 2019 to explore vectorising their LRRK2 inhibitors, suggesting that while the initial proof-of-concept test for LRRK2 inhibition is focused on oral treatment, the long-term future may be more focused on gene therapy approaches (Source).
Another interesting note from the Biogen/Denali collaboration is where Biogen’s other LRRK2 inhibition program stands.
 Working in collaboration with the biotech firm Ionis Pharmaceuticals, Biogen has been developing a different kind of LRRK2 inhibition approach.
Working in collaboration with the biotech firm Ionis Pharmaceuticals, Biogen has been developing a different kind of LRRK2 inhibition approach.
The companies have been working on BIIB094 – an antisense oligonucleotide targetting LRRK2. Antisense oligonucleotides are a method of inhibiting RNA rather than proteins – this means that this drug blocks LRRK2 RNA rather than the subsequent protein (Click here to read a previous SoPD post about this approach).
A Phase I clinical trial of BIIB094 was registered in late 2019. Called the “REASON study”, it involves 82 participants being recruited from 15 research centers in North America, Spain, Norway, the U.K., and Israel. The study is scheduled to complete until September 2023 (Click here to read more about this study).
|
Additional developments to look out for in 2022 regarding LRRK2-targeting agents: Small molecule inhibitors:
Antisense oligonucleotide approaches for LRRK2:
RNA editing approaches for LRRK2:
|
In addition to alpha synuclein and LRRK2, another direct approach for slowing the progression of Parkinson’s is focused on an enzyme that is involved with the waste disposal/recycling system of cells:
The direct approach: GBA
Genetic variants in the GBA gene are among the most common risk factors for Parkinson’s.
The GBA gene provides the instructions for producing an enzyme called Glucocerebrosidase (or GCase). This enzyme helps to break down glucocerebroside (into glucose and ceramide) in the lysosome (Click here to read a previous SoPD post about this).
 Source: Prevail
Source: Prevail
In people with GBA genetic variants, it is believed that the GCase enzyme is not functioning correctly which results in aggregation of alpha synuclein protein and cell death. These individuals typically have an earlier onset of PD and a faster progression (although this can vary considerably between cases).
As a result of the association between GBA and Parkinson’s, a great deal of research has been conducted on the biology of this particular pathway, with multiple clinical trial programs now testing GBA-based therapies.
In 2020, we saw the results of the “Ambroxol in Disease Modification in Parkinson Disease” (or AIM-PD) clinical trial clinical trial (Click here to read a SoPD post on these results).

Ambroxol. Source: Skinflint
This trial was supported by Cure Parkinson’s Trust, and partners: the Van Andel Research Institute (USA) and the John Black Charitable Foundation.
Ambroxol is a commonly used treatment for respiratory diseases. It promotes the clearance of mucus and eases coughing. It also has anti-inflammatory properties, reducing redness in a sore throat. But there is considerable evidence that this drug can also increase the levels of the GCase protein in models of Parkinson’s (Click here to read a SoPD post on this).
The results of the AIM-PD study indicated that ambroxol was able to elevate levels of GCase in the brains of the participants:
 Source: JAMA
Source: JAMA
A larger, longer Phase III clinical trial is now in development to evaluate the efficacy of ambroxol in Parkinson’s. Details of this study will be announced in 2022.
There is also a second Ambroxol study, which is being conducted in London, Canada. This is a phase II, 52 week trial of ambroxol in 75 people with Parkinson’s Disease Dementia (Click here to read more about this trial). In this randomised, double blind study, two doses of ambroxol were tested – a high dose (1050 mg) and a low dose (525 mg) – as well as a placebo treated group. This study was scheduled to finish in December 2021, so we will be looking for news regarding the results of this study in 2022.
One set of GBA-related clinical trial results we were looking out for in 2021 came from the “MOVES-PD” study of Venglustat (formerly known as GZ/SAR402671 & Ibiglustat) which was conducted by the biotech company Sanofi Genzyme.
This was a phase II clinical study that will be involve in two parts (Click here to read more about the trial):
- A dose escalation study to determine safety in early-stage GBA-associated Parkinson’s.
- A randomised, double blind study of efficacy of Venglustat, as compared to placebo in early-stage GBA-associated Parkinson’s.
Venglustat is an experimental agent that reduces the levels of the protein that GCase breaks down. Specifically, it inhibits a enzyme called glucosylceramide synthase. The preclinical results of this treatment approach looked promising (Click here to read some of the research on this).
But then in February 2021, Sanofi announced in a single sentence of their annual report that “the venglustat Phase 2 trial in Parkinson’s with GBA mutations did not meet the primary endpoint (end-January) & the indication was halted” (Click here to read more).
The safety & PK/PD data from the MOVES-PD study have been published (Click here to read that report), but we are still waiting on the final results of the study.
On a more positive, one clinical trial we will be looking forward to learning more about in 2022 is the Bial Biotech.
In 2020, the biotech firm Lysosomal Therapeutics (LTI) was acquired by the Portuguese pharmaceutical company Bial. LTI have completed Phase I clinical studies of their experimental drug LTI-291, which is an activator of the GCase enzyme (Click here to read a previous SoPD post on this topic). A new company has been set up – BIAL Biotech – and it is now seeking to further develop LTI-291 (also known as BIA 28-6156), but before the next phase of clinical testing can be conducted, long term toxicology studies have been required. We are hoping to see a large scale clinical evaluation of LTI-291 starting in 2022.
In addition to small molecule agents targeting GBA, there are also some companies exploring gene therapy approaches. In 2020, the gene therapy firm Prevail Therapeutics was acquired by the pharmaceutical company Eli Lilly (Click here to read about this).
This company is conducting the “PROPEL” trial in GBA-associated Parkinson’s with the aim of introducing a normal version of the GBA gene into the brain (via AAV9 viral vectors; the treatment is called PR001), allowing the cells to correct any lysosomal disfunction (Click here to read more about this trial). This trial does not complete until 2027, but we will hopefully get some initial updates on progress with this trial in 2022.
Another company developing a GBA-based gene therapy approach is AVROBIO.
 The company is focused on Type 1 Gaucher disease at present, but have indicated that their gene therapy treatment (called AVR-RD-02) could be used in Parkinson’s (Source). AVR-RD-02 is a lentiviral based gene therapy, which is currently in Phase I testing in Type 1 Gaucher disease (Click here to read more about that trial). That study is scheduled to finish in December 2023.
The company is focused on Type 1 Gaucher disease at present, but have indicated that their gene therapy treatment (called AVR-RD-02) could be used in Parkinson’s (Source). AVR-RD-02 is a lentiviral based gene therapy, which is currently in Phase I testing in Type 1 Gaucher disease (Click here to read more about that trial). That study is scheduled to finish in December 2023.
In addition to these GCase-specific approaches, there are also a number of other GBA-associated PD agents being developed.
In 2022, we will be watching out for the initiation of a new clinical trial for a drug called ESB1609, which is being developed by E-scape Bio (which also has a LRRK2 inhibitor program mentioned above).
 ESB1609 is a novel, orally administered, brain-penetrant, selective sphingosine 1-phosphate 5 (S1P5) receptor agonist. S1P5 provides a powerful target that is upstream to some of the autophagy-related (lysosomal) deficits associated with conditions like GBA-associated Parkinson’s (Click here to read more about this). The first clinical trial was a single dose study, and the company announced good safety results in early 2020 (Click here to read more about this). They were seeking to initiate further dosing studies in 2020, but this may have been delayed by COVID. We will be looking for an update on this drug in 2022 – it is being developed for Niemann-Pick C and GBA-associated Parkinson’s.
ESB1609 is a novel, orally administered, brain-penetrant, selective sphingosine 1-phosphate 5 (S1P5) receptor agonist. S1P5 provides a powerful target that is upstream to some of the autophagy-related (lysosomal) deficits associated with conditions like GBA-associated Parkinson’s (Click here to read more about this). The first clinical trial was a single dose study, and the company announced good safety results in early 2020 (Click here to read more about this). They were seeking to initiate further dosing studies in 2020, but this may have been delayed by COVID. We will be looking for an update on this drug in 2022 – it is being developed for Niemann-Pick C and GBA-associated Parkinson’s.
Another GBA-associated Parkinson’s clinical program is being developed by PTC Therapeutics.
The company is developing PTC857, which is an inhibitor of 15-Lipoxygenase – this is an enzyme that is a key regulator of the oxidative stress, protein aggregation and inflammation response pathways. The company originally stated that PTC857 was being developed for GBA-associated Parkinson’s (Source). But since completion of Phase I testing in 2021, they appear to have shifted indication to ALS (Source). We will be curious to see if they shift back.
|
Additional developments to look out for in 2022 regarding GBA-targeting agents:
|
In addition to alpha synuclein, LRRK2, and GBA there are a number of other Parkinson’s associated proteins and pathways that believed to be playing a ‘direct’ role in the progression of Parkinson’s. Many of these revolve around the activity of mitochondria.
The direct approach: Mitochondria
Mitochondria are the power stations of each cell. They help to keep the lights on. Without them, the party is over and the cell dies.

Mitochondria and their location in the cell. Source: NCBI
When mitochondria are old or damaged, they will start to release messenger proteins to alert the cell of their state. This will initiate a process of removing/disposing of the affected mitochondria – that process is called mitophagy. If too many mitochondria start excreting messenger proteins, however, the cell will become overwhelmed and die.
Mitochondrial dysfunction has long been associated with Parkinson’s. In addition, genetic mutations in several genes involved with the process of removing old/damaged mitochondria (mitophagy) have been associated with a higher risk of developing Parkinson’s (and this is why “mitochondria” are being considered a “direct approach” to component 1).
As a result, researchers have been developing therapies that are focused on improving mitochondrial function. By doing this, the theory is that the mitochondria will be healthier and able to better support the cell. Healthier cells will hopefully lead to a slower progression of Parkinson’s.
 Mitochondria (gold) within cells with blue nuclei
Mitochondria (gold) within cells with blue nuclei
One of the big events we will be looking out for this year in Parkinson’s research is the announcing of the UP study results.
In February 2019, a clinical trial was initiated to evaluate UDCA (aka Ursodeoxycholic acid or ursodiol) in Parkinson’s. UDCA is a clinically-available medication for the treatment of gallstones and liver disease, and it is being repurposed for Parkinson’s based on preclinical research suggesting that it has beneficial effects on mitochondrial function. This property led to improvements in models of Parkinson’s, which resulted in the development of the “UP” study (“UDCA in Parkinson’s” study – Click here to read a SoPD post on the topic).
The study – involving 30 participants who are less than 3 years since diagnosis – was a Phase II, placebo-controlled, double blind, randomised clinical trial, which was assessing the safety and tolerability of 30 mg/kg daily dosing of UDCA in Parkinson’s. The study was completed in 2021, and we will find out the results in 2022.
Cure Parkinson’s is a supporter of the UP study.
There was a second clinical trial of UDCA in Parkinson’s, which was conducted at the University of Minnesota.
It was a Phase I open label study was designed to assess the safety/tolerability of increasing doses of UDCA (Click here to read more about this). That study published its results in 2020, and UDCA was found to be safe and well tolerated at the doses used (Click here to read a SoPD post on this topic). It will be interesting to learn of any plans for future clinical development of UDCA in 2022.
In 2022, we also hope to learn of future plans for another mitochondrial-targeted drug called CNM-Au8, which is being developed by a company called Clene Nanomedicine.
 This gold-derived (seriously) treatment acts as a potent anti-oxidant, but it also boosts the energy production in mitochondria (Click here to read an SoPD post about this research).
This gold-derived (seriously) treatment acts as a potent anti-oxidant, but it also boosts the energy production in mitochondria (Click here to read an SoPD post about this research).
In August 2021, the results of the REPAIR-PD study were announced (Source). The study confirmed that the treatment was safe and well tolerated and also found consistent brain target engagement in people with Parkinson’s. Importantly, the company showed catalytic bioenergetic improvements in the brain, which is encouraging to hear as they now look towards initiating a large, double blind study (Called RESCUE-PD) in the first half of 2022 (Source).
FAScinate Therapeutics (a subsidiary of the South Korean Biotech company Kainos Medicine) announced in November 2021 that they had been cleared for Phase II clinical testing of KM-819 – a small molecule inhibitor for FAF1 (Source).
FAF1 is a protein that can cause mitochondrial dysfunction (via JNK1 activation) and is involved with instructing cells to die, so the researchers are investigating whether inhibiting/blocking FAF1 could be beneficial in slowing the progression of Parkinson’s.
Kainos completed a Phase I clinical study in 2019 evaluating KM-819 in a randomised, double-blind, placebo-controlled dose-escalation study in healthy volunteers, that found that the drug was safe and tolerable with no drug-related SAEs. The results of that study have been published (Click here to read the results of that study). We will be looking for a large Phase II clinical trial to be initiated in 2022.
In September 2019, a research report was published that indicated that the prostatic hyperplasia and hypertension drug, Terazosin, had beneficial effects in models of Parkinson’s (Click here to read an SoPD post on this topic).
 Researchers found that terazosin could rescue models of Parkinson’s by boosting energy production in mitochondria. A 12 week Phase II clinical trial for Terazosin in Parkinson’s was subsequently set up to assess safety of the drug in people with Parkinson’s (Click here to read about the clinical trial). The results of that study were reported in 2021 (Click here to read a SoPD post on this topic).
Researchers found that terazosin could rescue models of Parkinson’s by boosting energy production in mitochondria. A 12 week Phase II clinical trial for Terazosin in Parkinson’s was subsequently set up to assess safety of the drug in people with Parkinson’s (Click here to read about the clinical trial). The results of that study were reported in 2021 (Click here to read a SoPD post on this topic).
The Michael J Fox Foundation is currently supporting a study exploring target engagement of terazosin in healthy volunteers (Click here to read more about that study). The goal of that study is to build up as much data as possible to help guide the design of the future studies investigating the progression limiting potential of terazosin in PD. This study will hopefully be providing results in 2022.
 There is also a new study looking at terazosin in individuals with Lewy body dementia. This is another pilot study, but it is looking to recruit 40 participants for for 15 weeks who will be treated with placebo or two different doses of terazosin (Click here to read more about the TZ-DLB study).
There is also a new study looking at terazosin in individuals with Lewy body dementia. This is another pilot study, but it is looking to recruit 40 participants for for 15 weeks who will be treated with placebo or two different doses of terazosin (Click here to read more about the TZ-DLB study).
A clinical trial of Nicotinamide Riboside (a form of Vitamin B3) has started in Norway – it is called the ‘NOPARK’ Study. Nicotinamide Riboside is an important component in energy production and mitochondrial function – we have previously discussed the biology of Nicotinamide Riboside (Click here to read that SoPD post).
 This study is a randomised, double-blind trial involving 400 participants with newly diagnosed Parkinson’s, who will be randomly assigned in an 1:1 ratio to either nicotinamide riboside or placebo treatment for 52 weeks. This study is scheduled to finish in March 2024 (Click here to read more about this study).
This study is a randomised, double-blind trial involving 400 participants with newly diagnosed Parkinson’s, who will be randomly assigned in an 1:1 ratio to either nicotinamide riboside or placebo treatment for 52 weeks. This study is scheduled to finish in March 2024 (Click here to read more about this study).
Another mitochondrial-targeting agent that I am hoping to hear news about in 2022 is EPI-589 (aka BioE-589) which is being developed by PTC Therapeutics (formerly BioElectron).
Preclinical data indicates that this drug helps to boost mitochondrial function. The company have conducted a Phase II open label, safety trial for the evaluation of EPI-589 in people with early onset genetic forms of Parkinson’s and also idiopathic Parkinson’s (Click here to learn more about this trial). And PTC had been planning further development – I’m assuming COVID has delayed plans.
And this agent is interesting given the announcement in late 2018 of positive results for an open label Phase II clinical trial of EPI-589 in motor neurone disease/ALS. That study was assessing safety, tolerability, and disease biomarker effect, and the results “provide a strong rationale for the continued development of EPI-589” in ALS (Click here to read more about this and click here for the details of that study).
A second compound that has exhibited interesting results in ALS is CuATSM which is being developed by Collaborative Medicinal Development Pty (Click here to read more about the ALS result).
CuATSM is a highly effective scavenger of a chemical in our bodies called ONOO, which can be very toxic. In addition, there is evidence that the drug also blocks the aggregation of alpha synuclein and has beneficial effects in models of Parkinson’s (Click here to read an example).
In 2019, we learnt the results of a small Phase I clinical trial of CuATSM in Parkinson’s. The study found that 24 weeks of treatment with the drug was well tolerated, and the participants experienced some improvements in their symptoms (Click here to read more about this). This was an open label study, and we are still waiting to see the results published. But it would be again encouraging to see progress on a larger, double blinded, placebo-controlled study started in 2022.
Yet another company that recently announced a positive result in ALS is Amylyx.
 In 2020, this biotech announced that their experimental combination therapy called AMX0035 had a significant effect in a double-blind clinical trial in ALS patients (Click here to read a SoPD post about this). AMX0035 which is a combination of sodium phenylbutyrate and tauroursodeoxycholic acid (TUDCA) and the company is keen to evaluate this drug in other neurodegenerative conditions. It would be interesting to see agent clinically tested in Parkinson’s in 2022.
In 2020, this biotech announced that their experimental combination therapy called AMX0035 had a significant effect in a double-blind clinical trial in ALS patients (Click here to read a SoPD post about this). AMX0035 which is a combination of sodium phenylbutyrate and tauroursodeoxycholic acid (TUDCA) and the company is keen to evaluate this drug in other neurodegenerative conditions. It would be interesting to see agent clinically tested in Parkinson’s in 2022.
|
Preclinical developments in mitochondrial research to look out for in 2022: Mitochondrial focused research programs:
PINK & PARKIN targeting agents:
Deubiquitinating (DUB) enzyme inhibitors: Deubiquitinating enzyme make the removal of damaged mitochondria more difficult. Inhibiting them is viewed as a means of stimulating mitophagy (Click here to read a SoPD post on this topic).
|
In addition to the categorised direct approaches listed above, there are a number of new direct methods that are being explored and which deserve mention here as things to look out for in 2022, so we will add another category here called:
The direct approach: Additionals
This category refers to therapies in development that explore novel mechanisms of action associated with the underlying biology of Parkinson’s.
The small biotech firm called CuraSen is taking a slightly different approach towards tackling Parkinson’s (Click here to read a SoPD post on this topic).
 Given the association of the locus coeruleus and the noradrenergic system in Parkinson’s, this struck me as a novel direct approach for slowing Parkinson’s (that did not fit nicely into any of the other categories).
Given the association of the locus coeruleus and the noradrenergic system in Parkinson’s, this struck me as a novel direct approach for slowing Parkinson’s (that did not fit nicely into any of the other categories).
In March 2021, the company announced Phase 1 proof-of-principle results demonstrating that salbutamol – a clinically available drug approved to treat respiratory disease – significantly improved brain perfusion as measured by increased cerebral blood flow in healthy subjects. (Click here to read more about this). And then in November 2021, Curasen reported that CST-103 (a beta-2 adrenoceptor agonist – similar to salbutamol) significantly increased regional cerebral blood flow in key areas of the brain in people with Parkinson’s (Source).
The company is currently conducting a Phase II randomized, placebo-controlled, crossover study with CST-103 in 40 patients with mild cognitive impairment, Parkinson’s with rapid eye movement sleep disorder (RBD) or Lewy body dementia. The results of this study should be available in the second half of 2022.
In 2021, the biotech firm Cortexyme announced a partnership with the Parkinson Study Group to form an Advisory Board and further develop the company’s Parkinson’s disease (PD) program.
 The company had been hoping to initiate a Phase II clinical trial of their gingipain inhibitor atuzaginstat, but in January 2022 they received a letter from the US FDA placing a full clinical hold on atuzaginstat activities. Hopefully the company will be able to resolve this matter to further their efforts in Parkinson’s (Click here to read a previous SoPD post on this topic).
The company had been hoping to initiate a Phase II clinical trial of their gingipain inhibitor atuzaginstat, but in January 2022 they received a letter from the US FDA placing a full clinical hold on atuzaginstat activities. Hopefully the company will be able to resolve this matter to further their efforts in Parkinson’s (Click here to read a previous SoPD post on this topic).
In 2022, we are hoping to see more clinical trials starting focused on novel mechanisms of action that take a ‘direct approach’ towards slowing, stopping or reversing Parkinson’s.
|
Preclinical developments in “The direct approach: Additionals” category to look out for in 2022:
|
This is where activities currently lie with regards to (what I have described as) the direct approach to slowing or halting Parkinson’s progression. As I wrote above, the direct approach involves treatments that specifically target the underlying biology of the condition. This suggests that a particular gene or biological pathway is associated with Parkinson’s.
We will now shift our attention to the:
The indirect approach
While the direct approach to halting disease progression is focused on what we know about the underlying biology of Parkinson’s, indirect approach does not.
In our discussion here, an indirect approach is one that does not necessarily target a protein or biological pathway that is directly associated with Parkinson’s, but rather it attempts to slow progression by improving the overall health of affected cells, and allowing them to function better in the face of whatever is driving Parkinson’s.
One way we can improve the health of cells (and potentially slow the progression of Parkinson’s) is to enhance their ability to clear (or dispose of) old and potentially toxic proteins. This approach generally involves boosting the waste disposal systems of the cell – in this manner, the cells can break down and dispose of excess proteins (like alpha synuclein) inside the cell before they have a chance to builds up and becomes toxic.
 Source: Nexcelom
Source: Nexcelom
A great deal of Parkinson’s research has focused on enhancing the cellular waste disposal/recycling process, which is referred to as autophagy.
The indirect approach: Autophagy
Helping cells to clean themselves up by boosting waste disposal systems, we will hopefully make the cells healthier and function better. And by limiting the build up of proteins – like alpha synuclein – these experimental therapies may help to slow down the progression of Parkinson’s.
As you shall see below, there are numerous clinical trials currently testing different therapies attempting to boost the autophagy process. One ‘autophagy boosting’ approach to slowing Parkinson’s involves a class of drugs called c-Abl inhibitors.
These molecules started life as cancer drugs, but they are now being re-purposed for Parkinson’s.
c-Abl is a protein that becomes activated in cells that are stressed and inhibiting it can boost autophagy. Multiple independent labs have demonstrated that this is a worthy target for Parkinson’s (Click here to read a review on this topic).
The first c-Abl inhibitor to be clinically tested in Parkinson’s was Nilotinib.
 Nilotinib. Source: William-Jon
Nilotinib. Source: William-Jon
Following evidence suggesting beneficial effects in models of Parkinson’s and a small open label Phase I pilot study (Click here to read an old SoPD post about this topic), two large double-blind Phase II clinical trials were initiated: PD Nilotinib and NiloPD.
PD Nilotinib, was conducted at Georgetown University in Washington DC (Click here for the more details about this study), and in late 2019 the investigators reported that the drug was safe at lower doses, but “no significant differences were seen in motor and nonmotor outcomes between the nilotinib groups and the placebo group” (Click here to read more about this).
The NILO-PD study was a multi-center study which also finished in 2019, and the results were published in 2020. The treatment was acceptable safety & tolerability, but data “indicate that nilotinib should not be further tested in PD“.
The researchers reported that “the low cerebrospinal fluid exposure & lack of biomarkers effect combined with the efficacy data trending in the negative direction indicate that nilotinib should not be further tested in Parkinson’s“. There was no difference in the clinical measures (change of MDS-UPDRS-3 OFF) from baseline to 6 months between the treatment groups. Cerebrospinal fluid/serum ratio of nilotinib concentration was 0.2% to 0.3% (Click here to read more about the results)
 Cure Parkinson’s was a supporter of the NILO-PD study.
Cure Parkinson’s was a supporter of the NILO-PD study.
These results have left a cloud hanging over c-Abl inhibitors as a potential therapeutic class, but (and I am happy to go on the record here – giving an actual opinion!) based on the extremely low levels of nilotinib that actually got into the brain in these studies (0.2% to 0.3% of the level in serum), I don’t think we have had a proper test of the c-Abl inhibition theory in Parkinson’s yet.
And I will not be satisfied until a brain-penetrant c-Abl inhibitor has been tested.
Luckily, numerous brain-penetrant c-Abl inhibitors are already in clinical trials for Parkinson’s.
Chief among these is Vodobatinib (formerly known as K0706), which is being developed by Sun Pharma Advanced Research Company (or SPARC).
 In 2019, SPARC initiated “PROSEEK” – a large international Phase II, randomised, double-blind, placebo-controlled clinical trial of vodobatinib in 500+ people with early Parkinson’s (Click here to read more about this study). This study is scheduled to finish in mid 2023.
In 2019, SPARC initiated “PROSEEK” – a large international Phase II, randomised, double-blind, placebo-controlled clinical trial of vodobatinib in 500+ people with early Parkinson’s (Click here to read more about this study). This study is scheduled to finish in mid 2023.
Cure Parkinson’s is a supporter of the PROSEEK study.
Another c-Abl inhibitor being targeted at Parkinson’s is FB-101, which is being developed by the biotech firm 1ST Biotherapeutics.
Phase I clinical testing of FB-101 in healthy volunteers has been conducted (Click here to read more about that study). The study was scheduled to finish mid 2020, so we will be looking for an update about this clinical program in 2022.
Two interesting things to note regarding 1stBio:
- They are also developing a PET tracer for c-Abl brain imaging (Source).
- They are collaborating with the biotech firm Neuraly to bring more c-Abl inhibitors to the clinic (Source).
In late 2020, we learned that another brain penetrant c-Abl inhibitor called Radotinib, entered clinical trial for Parkinson’s. This agent is being developed by South Korean firm Ilyang Pharmaceutical.
![]() Radotinib is currently in a Phase II randomized double-blind, placebo-controlled study involving 40 participants. The trial is scheduled to finish in April 2022 (Click here to read more about this trial).
Radotinib is currently in a Phase II randomized double-blind, placebo-controlled study involving 40 participants. The trial is scheduled to finish in April 2022 (Click here to read more about this trial).
And another biotech company – Inhibikase Therapeutics – has started Phase I testing their c-Abl inhibitor for Parkinson’s.
The agent is called IkT-148009 and researchers involved with this company recently published a very nice review of the research supporting c-Abl inhibition for Parkinson’s. A Phase I trial of IkT-148009 is being conducted in 112 healthy elderly volunteers (Click here to read more about this), and in October 2021, the company announced that they had dosed the first Parkinson’s patient in their Phase Ib study (Click here to read more about this). This study is scheduled to finish in December 2022. But Inhibikase Therapeutics is hoping to initiate “a Phase 2a study in 2022” (Source).
|
Preclinical developments in autophagy research to look out for in 2022:
|
Another indirect approach to slowing the progression of Parkinson’s focuses around anti-inflammatory approaches:
The indirect approach: Anti-inflammatory approaches
When cells in your body are stressed or sick, they begin to release tiny messenger proteins which inform the rest of your body that something is wrong. When enough of these messenger proteins are released that the immune system becomes activated, it can cause inflammation.
Inflammation is a critical part of the immune system’s response to trouble. It is the body’s way of communicating to the immune system that something is wrong and activating it so that it can help deal with the situation.
By releasing the messenger proteins (called cytokines), injured/sick cells kick off a process that results in multiple types of immune cells entering the troubled area of the body and undertaking very specific tasks.
 The inflammatory process. Source: Trainingcor
The inflammatory process. Source: Trainingcor
There is now a great deal of evidence to suggest that inflammation is playing a role in neurodegenerative conditions like Parkinson’s. As a result, researchers have been exploring methods of reducing the immune response in an effort to restrict the potential damage inflicted. This has led to a number of clinical trials.
In 2021, one such anti-inflammatory clinical trial was initiated here in the UK. Researchers at Cambridge University initiated the testing the immunosuppressive medication, Azathioprine, in people with Parkinson’s (Click here to read more about this trial). The “AZA-PD study” is a phase II randomised placebo-controlled double-blind trial in 60 individuals with recently diagnosed Parkinson’s.
 Cure Parkinson’s is a supporter of the AZA-PD study.
Cure Parkinson’s is a supporter of the AZA-PD study.
The participants in this study will be treated with azathioprine (or placebo) for 12 months, and then there will be a further 6-month follow-up assessment (Click here to read more about this study).
The South Korean biotech firm GNT Pharma has conducted a Phase I trial for their anti-inflammatory drug called Crisdesalazine (Click here to read more about this).
 Crisdesalazine (also known as AAD-2004) is an inhibitor of microsomal prostaglandin E2
Crisdesalazine (also known as AAD-2004) is an inhibitor of microsomal prostaglandin E2
synthase-1 (mPGES-1) that also has reactive oxygen species scavenging actions. Safety
was verified in the Phase I study via treatment across 4 cohorts (20, 50, 100, and 200 mg), but no details on the results have been released to the research community
Another inflammation-related biotech company that we are watching in 2022 is Inmune Bio.
This biotech firm is developing a drug called Xpro™ (formerly Xpro1595), which targets soluble TNF. Tumor Necrosis Factor (TNF) is a potent immune signaling molecule (a cytokine) and it is intimately involved in inflammation. But Xpro is different to current clinically approved TNF inhibitors, as it only neutralises soluble TNF, while not affecting trans-membrane TNF (this is an important difference).
Inmune Bio has conducted a Phase I clinical study of XPro in individuals with moderate Alzheimer’s (Click here to read more about this trial). In September 2021, the company announced that once a week treatment with XPro for three months significantly reduced specific markers in the brain and improved white matter MRI metrics (in brain imaging – Click here to read more about this).
The company was planning to start enrolling participant in a blinded, randomized Phase II cllinical trial of Xpro in patients with mild Alzheimer’s in late 2021. This six-month study will enroll 200 participants at research centers across North America and Australia. Fingers crossed the company also considers testing the drug in Parkinson’s.
In 2022, I am also hoping to hear news about research related to sargramostim.
 In 2017, researchers in Nebraska completed a clinical trial assessing this immunomodulator drug in Parkinson’s, and they reported interesting results (Click here to read a SoPD post about this). It was suggested by the investigators conducting that study, however, that a reformulation of the drug was required as many of the participants developed antibodies to the drug (which could potentially render the therapy useless by blocking its action).
In 2017, researchers in Nebraska completed a clinical trial assessing this immunomodulator drug in Parkinson’s, and they reported interesting results (Click here to read a SoPD post about this). It was suggested by the investigators conducting that study, however, that a reformulation of the drug was required as many of the participants developed antibodies to the drug (which could potentially render the therapy useless by blocking its action).
In 2021, the researchers published the results of a follow-up study involving 5 individuals taking a lower dose of sargramostim for a year. This was a small Phase Ib biomarker and safety study, but the researchers did report that “MDS-UPDRS Part III scores did not worsen” (Click here to read more about this). In addition, they also published preclinical research on a novel molecule – called PDM608 – which displays better properties than sargramostim with the neuroprotective benefits as well (Click here to read more about this). It will be interesting to see how this research develops in 2022.
In August 2020, Spanish multinational pharmaceutical company Grifols to acquired a small biotech called Alkahest (Source).
 Alkahest had been developing an orally administered CCR3 inhibitor AKST4290. CCR3 is the natural receptor for eotaxin – an immunomodulatory chemokine that is increased in normal aging as well as multiple diseases of aging. It has been implicated in Alzheimer’s, Parkinson’s, and other aging-related diseases that involve inflammation.
Alkahest had been developing an orally administered CCR3 inhibitor AKST4290. CCR3 is the natural receptor for eotaxin – an immunomodulatory chemokine that is increased in normal aging as well as multiple diseases of aging. It has been implicated in Alzheimer’s, Parkinson’s, and other aging-related diseases that involve inflammation.
In 2020, the company initiated a Phase II clinical trial of AKST4290 in 110 people with Parkinson’s. Participants received 400mg AKST4290 or placebo pills twice daily for 12 weeks (Click here to read more about this clinical trial). This trial completed in 2021 so we will hopefully learn the results of it in 2022.
And while not specifically an alpha synuclein inhibitor, NeuaroPore Therapies is working on a drug called NPT520-34 which has anti-aggregation properties.
They completed Phase I clinically testing of NPT520-34 in healthy individuals in September 2019 (Click here to read more about this trial). In January 2020, the company announced that “The results of this study support moving forward to a safety study in patients. Our team is currently evaluating the optimal study design and patient population for the next study” (click here to read that press release). COVID-19 may have delayed progress, but the company has been publishing preclinical data related to this molecule (Click here to read more about this). We will be looking out for news of future development in 2022.
The biotech company BioVie recently announced the dosing of their first participant in a Phase 2 clinical trial of their lead molecule for Parkinson’s (Source).
NE3107 is an ERK inhibitor that selectively inhibits neuroinflammation & insulin resistance. BioVie is developing NE3107 for Alzheimer’s and Parkinson’s (Source). The topline results of the Phase 3 trial in Alzheimer’s are expected at the end of 2022.
The new Parkinson’s trial is a 28-day Phase 2a, double-blind, placebo-controlled (1:1) study evaluating 20 mg NE3107 twice daily in 40 individuals with Parkinson’s (taking 400 mg L-dopa daily). This study is also scheduled to complete at the end of 2022 (Click here to read more about this trial).
One area of inflammation-related research that is very hot at the moment is efforts to inhibit the inflammasome.
Inflammasomes are multi-protein formations, present inside of cells in your body, and they can amplify the immune response to damage or a pathogen. Recent preclinical research suggests that blocking the inflammasome can rescue models of Parkinson’s (Click here to read a SoPD post on this topic)
Researchers have also reported that the Parkinson’s-associated protein alpha synuclein can promote the activation of inflammasomes in the immune cells of the brain: the microglia.
This has given rise to the development of NLRP3 inhibitors (NLRP3 is a key protein involved in inflammasomes). The first of these inhibitors to reach the clinic is Inzomelid, which was being developed by a biotech firm called Inflazome.
 Phase I clinical studies of Inzomelid were completed in early 2020 (click here to read more about those studies) and the drug was found to be safe and well tolerated in double-blind evaluations in healthy volunteers (Click here to read more about this).
Phase I clinical studies of Inzomelid were completed in early 2020 (click here to read more about those studies) and the drug was found to be safe and well tolerated in double-blind evaluations in healthy volunteers (Click here to read more about this).
Then in September 2020, it was announced that Inflazome was being acquired by the pharmaceutical company Roche:
 Roche appears to be planning to advance inzomelid across multiple inflammation-based indications, starting with a Phase II study for CAPS being conducted in the UK (Source).
Roche appears to be planning to advance inzomelid across multiple inflammation-based indications, starting with a Phase II study for CAPS being conducted in the UK (Source).
The future development of inzomelid will also include Phase II studies for Parkinson’s, and those trials will most likely make use of an NLRP3-targeted PET imaging tracer, that Inflazome has developed with funding from the Michael J. Fox Foundation (Click here to read more about this). We have not had any news regarding the future development of inzomelid, but one of the researchers (Prof Luke O’Neill) behind Inflazome made an interesting comment last year in this video (watch from the 12 minutes mark – thanks to William for spotting this):
Hopefully in 2022 we will learn more about the “6 clinical trials” that Roche has planned for the Inflazome NLRP3 inhibitors.
Another inflammasome focused biotech company is NodThera.
 In November 2021, this company announced that they had dosed the first participant in Phase I clinical testing of their lead development candidate, NT-0796 (Click here to read more about this). And they are developing other small molecule NLRP3 inhibitors that can cross the blood brain barrier with Parkinson’s and other neurodegenerative conditions in mind.
In November 2021, this company announced that they had dosed the first participant in Phase I clinical testing of their lead development candidate, NT-0796 (Click here to read more about this). And they are developing other small molecule NLRP3 inhibitors that can cross the blood brain barrier with Parkinson’s and other neurodegenerative conditions in mind.
|
Preclinical developments in inflammation research to look out for in 2022: Anti-inflammatory approaches:
Inflammasome targeting therapies:
|
In addition to these inflammation targeting approaches, there are other indirect approach treatments being clinically developed for slowing the progression of Parkinson’s.
The indirect approach: Gastrointestinal system
Over the last decade the role of the gut in general health has received a lot of attention with the acknowledgement that the bacteria in our gastrointestinal system is doing more than simply digesting our food.
Increasingly, we are discovering that these tiny organisms that live in symbiosis with us may also play an influential role in medical conditions such as Parkinson’s, and this has resulted in a number of clinical trials evaluating various approaches to try and adapt (or exploit) the potential of the “gut microbiota” (the communities of bacteria living in the 28 feet of our gastrointestinal tract).
One obvious way of manipulating the communities of bacteria in our guts is to alter them with the use of antibiotics.
The MICRO-PD (“Microbiota Intervention to Change the Response of Parkinson’s Disease”) study was scheduled to finish in the second half of 2021 (Click here to read more about this study). This Phase I/II study – which is being conducted in California – involves 86 participants who are being treated with the antibiotic Rifaximin. We should hopefully learn the results of this study in 2022.
Another method of adjusting the composition of the flora of the gut is via dietary adjustment, and there have been studies exploring this as well.
Resistant maltodextrin is a dietary fiber that enhances gut health, and this has been clinically tested recently in Parkinson’s. In a 4-week, Phase II study involving 30 individuals, the safety and tolerability of this treatment has been assessed (Click here to read more about this study). This study was also scheduled to complete in the middle of 2021, so maybe this year we will learn the results.
There are several clinical trials evaluating probiotics – live microorganisms that are ingested.
The Symprove study here in the UK is evaluating the impact of a probiotics on 60 individuals with Parkinson’s, assessing symptoms, inflammation and the gut microbiota itself (Click here to read more about this study). That study was scheduled to complete in 2020, and recently a publication related to this trial was published (click here to read more about this), but the results of the study are still undisclosed. We will hopefully have an update in 2022.
Another probiotic study called the TAP (“Treating Anxiety in Parkinson’s Disease With a Multi-Strain Probiotic”) study is focused more on symptom response to a probiotic treatment (called Ecologic® BARRIER 849) but they are also assessing clinical measures of progression (Click here to read more about this study). For a short summary of the study – click here. This study is scheduled to finish in December 2022.
Not to be confused with fetal cell transplantation (which will be discuss below in component #3 below), fecal transplantation involves attempting to introduce bacteria from the gastrointestinal system of “healthy” individuals into the gastrointestinal system of people with medical conditions like Parkinson’s.
There have been two recent trials on fecal transplantation in Parkinson’s.
The first of them was a Phase IIa study being conducted in Israel. It involved 10 participants who were evaluated for 6 months in a open label fashion (Click here to read more about this trial). The results of the study were published in 2021 and they indicated that fecal transplants (infused via colonoscopy) were safe and improved of motor and non-motor symptoms (including constipation – Click here to read more about this).
And the second equally small fecal transplant study is being conducted in Houston (Texas). It involves 12 participants who are being evaluated over 12 weeks following treatment with an oral encapsulated fecal transplant called PRIM-DJ2727 (Click here to read more about this trial). This study is scheduled to be completed in November 2022.
One piece of news that we will be looking for in 2022 is a progress report from Leeds-based biotech firm 4D Pharma, which is developing live biotherapeutic treatments – including MRx0029 and MRx0005 – for neurodegenerative conditions.
The company is hoping to begin first-in-human Phase I/II clinical studies for both MRx0029 and MRx0005 in Parkinson’s in 2022 (Source). Some preclinical data demonstrating neuroprotection from these agents has been published (Click here to read more about this). 2022 will hopefully be an exciting year for the research team at 4D Pharma.
|
Preclinical developments in gastrointestinal system research to look out for in 2022: Small molecules:
Gut microbiota-derived therapies:
|
While it is essential for normal health, iron does start to accumulate with age. In many cases of Parkinson’s, this process appears to be exaggerated in certain regions of the brain, and as a result researchers have tested different methods of reducing excess iron levels in models of Parkinson’s as a method of slowing down the progression of the condition (Click here to read a review on this topic).
Indirect approach: Iron chelation
Several clinical trials have recently been conducted in Parkinson’s cohorts that are exploring the use of iron chelators – this is a class of medications that reduce the levels of iron in the body.
 Iron is known to accumulate in regions of the brain that are vulnerable in people with Parkinson’s, and so researchers have been evaluating iron chelators as a potential disease modifying therapy for Parkinson’s.
Iron is known to accumulate in regions of the brain that are vulnerable in people with Parkinson’s, and so researchers have been evaluating iron chelators as a potential disease modifying therapy for Parkinson’s.
In 2022, we are going to learn the results of one clinical trial of an iron chelator, which is called Deferiprone. This trial is called the FAIRPARK II study.
This is a large trial involving 330+ participants who are being treated with either deferiprone or a placebo treatment for 9 months (Click here to read more about this). It will be interesting to learn the results of this study in 2022.
Cure Parkinson’s is a supporter of the FAIRPARK II study.
A second deferiprone study in Parkinson’s was scheduled to finish in late 2019. This study (called the “SKY study”) recruited 140 people with early Parkinson’s and treated them twice-daily for 9 months with Deferiprone (one of 4 different doses) or a placebo drug (Click here to read more about the details of this study). We will hopefully learn the results of this trial as well in 2022. This international trial is being conducted by ApoPharma.
In 2018, Alterity Therapeutics (formerly PRANA Biotechnology) initiated a clinical trial of their iron chelator drug PBT434 in healthy individuals.
This was a Phase I study evaluating the safety, tolerability and pharmacokinetics of this treatment, after single and multiple oral dose administration (Click here to read more about that study and click here for a previous SoPD post on this topic).
In December 2021, the company received authorization from the New Zealand health authority to initiate a Phase 2 clinical trial for ATH434 in early stage Multiple System Atrophy (MSA, which is similar to Parkinson’s – Source). The double-blind clinical trial is a three-arm study, involving a low dose and high dose arm, as well as a placebo treated group. Participants will receive treatment for 12 months, and clinical endpoints and other biomarkers will be assessed. It would be interesting to see a Parkinson’s trial being conducted in parallel to this study in 2022.
|
Preclinical developments in iron chelation research to look out for in 2022:
|
Now we a going to shift gears slightly.
If one of these ‘direct’ or ‘indirect’ approaches is found to be able to slow or halt the progression of PD, the next requirement in a ‘curative therapy’ for Parkinson’s is:
COMPONENT #2. A neuroprotective agent
Once a drug or a treatment has been determined to slow down the progression of Parkinson’s, it will be necessary to protect the remaining cells and provide a nurturing environment for the third part of the ‘cure’ (Cell replacement therapy – more on that below).
Neuroprotection is the area of research that has had the most attention over the years. Drug companies have employed vast resources in this area in the hope of discovering a treatment which will work across conditions (think Alzheimer’s, Parkinson’s, Huntington’s, etc), and thus provide them with tremendous profits. Unfortunately, conditions of the brain have proven to be a lot more complicated than first perceived and cross-condition therapies seem unlikely as we move towards greater stratification and personalisation of disease and treatment, respectively.
But there has been the hint of a potential neuroprotective effect in one class of drugs for Parkinson’s: GLP-1R agonists.
Neuroprotective approach: GLP-1R agonists
Exenatide is a glucagon like peptide-1 receptor (or GLP-1R) agonist. This is a class of drug that has traditionally been used for treating diabetes, but has recently been repurposed for Parkinson’s.
After multiple studies suggested neuroprotective properties in models of Parkinson’s, a clinical trial program was intiated, and in 2017, a Phase II Exenatide trial reported the stablisation of Parkinson’s motor features over the course of the 48 week trial (Click here and here to read previous SoPD posts about this).
The researchers found a statistically significant difference in the motor scores of the exenatide-treated group verses the placebo group (p=0·0318). As the placebo group continued to have an increasing (worsening) motor score over time, the exenatide-treated group demonstrated improvements, which remarkably remained after the treatment had been stopped for 3 months (weeks 48-60 on the graph below).
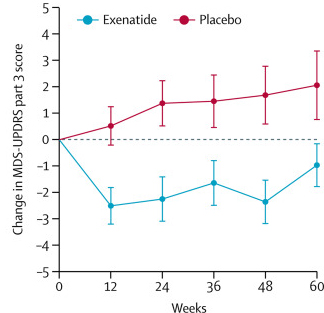
Reduction in motor scores in Exenatide group. Source: Lancet
Brain imaging (DaTScan) also suggested a trend towards reduced rate of decline in the exenatide-treated group when compared with the placebo group. Interestingly, the researchers found no significant differences between the exenatide and placebo groups in scores of cognitive ability or depression – suggesting that the positive effect of exenatide may be specific to the dopamine or motor regions of the brain.
In late 2020, researchers involved in the 2017 study published an interesting report demonstrating that people with diabetes who had been treated with exenatide had a reduced risk of developing Parkinson’s (compared to those diabetics who were not treated with exenatide – click here to read that report and click here to read a SoPD post on this topic)
This and other results have led to a number of research groups and biotech companies developing GLP-1R agonists for Parkinson’s and initiating clinical trials. In 2022, we will see a number of these clinical trials either completing or reporting results.
In late 2019, we saw the initiation of a Phase III clinical trial for Bydureon/Exenatide.
The Phase III trial will involved 200 participants being treated for 2 years and is scheduled to complete in 2024 (Click here to read more about this trial and click here to see the trial webpage).
Cure Parkinson’s is a supporter of the Exenatide III study.
There is also a clinical trial evaluating exenatide in people with recently diagnosed Parkinson’s in Sweden. This is a Phase II study that has recruited 60 participants and follow them for 18 months. The study is scheduled to finish in October 2022 (Click here to read more about this study).
The University of Florida also has a small clinical trial evaluating 1 year of exenatide treatment in people with Parkinson’s. This study is scheduled to finish in May 2022 (Click here to read more about this study).
In addition to these three studies of exenatide, there are additional ongoing clinical trials for other GLP-1R agonists. For example, there has been the Lixisenatide trial in France, which recruited 156 participants to be treated with this GLP-1R agonist (or placebo) for 12 months. This study will be reporting results in 2022 (Click here and here for more information on this study).
And there has also been the Liraglutide trial in California, which involved 60 individuals with PD being treated for 12 months. This study will also be reporting results in 2022 (Click here and here to read more about this study).
 Cure Parkinson’s is a supporter of both the Lixisenatide and Liraglutide studies.
Cure Parkinson’s is a supporter of both the Lixisenatide and Liraglutide studies.
In addition, there is a clinical trial of a new version of exenatide called Semaglutide in Parkinson’s. This study is being conducted by researchers in Norway who are accessing the drug via the Danish biotech firm Novo Nordisk.
This Phase II study will involve 120 participants, who will take either Semaglutide (or placebo) for 24 months in a double blind fashion, before a further 24 months of open label assessments. The study is scheduled to finish in 2024 (Click here to read more about this study).
Biotech firm Neuraly initiated a Phase II trial of their GLP-1R agonist NLY01 in February 2020. That study is a Phase II 36-week, placebo controlled, double-blind trial involving 240 participants, and it is scheduled to complete in August 2022 (Click here to learn more about that study).
Another biotech company developing a new GLP-1R agonist is Peptron.
This South Korean biotech firm have also conducted Phase I safety testing of their GLP-1R agonist (called PT320 – click here to read more about that trial). In 2020, Peptron initiated a Phase IIa study to evaluate the efficacy and safety of 48 weeks treatment with PT320 in 100 individuals with early Parkinson’s. This study was scheduled to complete in late 2021 (Click here to read more about that trial). We will hopefully learn the results of this study in 2022.
|
Preclinical developments in GLP-1R agonists research to look out for in 2022:
|
Another form of ‘neuroprotective’ treatment that has been tested in Parkinson’s for a long time is neurotrophic factors.
Neuroprotective approach: Neurotrophic factors
Neurotrophic factors are supportive/nurturing proteins that the brain produces naturally. They help to keep cells alive by activating and stimulating specific biological pathways. There are different types of neurotrophic factors which affect different types of neurons in different ways (one of my pet peeves is repetition of a word in a single sentence!).
 Source: Frontiers
Source: Frontiers
Glial cell-derived neurotrophic factor (GDNF)
One neurotrophic factor that has a particularly robust effect on dopamine neurons (the type of neurons most severely affected by Parkinson’s) is GDNF (or Glial cell-derived neurotrophic factor).
In 2019, we saw the publication of the Bristol Phase II GDNF clinical trial results. GDNF is a neurotrophic factor that has had a long roller coaster-like history with Parkinson’s. The results of the Phase II trial indicate that there was no significant improvement in those individuals being treated with GDNF compared to those on the control/placebo treatment (as measured by clinical rating scales – click here to read a SoPD post about these results).
Cure Parkinson’s was a supporter of the Bristol GDNF study.
Despite the set back with that GDNF trial, in February 2021 Parkinson’s UK announced that they were investing £800,000 over the next 2 years to try and make another clinical trial happen (Source).
Other groups are taking different approaches towards GDNF. For example, there is another GDNF trial that is investigating a gene therapy form of GDNF. A biotech firm called Brain Neurotherapy Bio published the results of a Phase I clinical trial assessing their AAV-based GDNF gene therapy treatment in people with Parkinson’s in 2019, and in 2020 this company was acquired by the pharmaceutical company Bayer (Click here to read a SoPD post on this topic).
The company is conducting a follow-up open-label safety study of their approach which was started in 2020 (Click here to read more about this trial). This trial will provide further case for support to conduct a much larger clinical trial. The initial results of this study are scheduled to complete in late 2022.
In late 2020, the pharmaceutical company Bayer acquired Brain Neurotherapy Bio (Click here to read a SoPD post on this topic) – yet another example of big pharma taking a strong interest in Parkinson’s.
 Another neurotrophic factor that is currently in clinical trial is CDNF:
Another neurotrophic factor that is currently in clinical trial is CDNF:
Cerebral dopamine neurotrophic factor (or CDNF).
This protein is very different to GDNF in its structure and mechanism of action, but has also been shown to have beneficial effects on dopamine neurons (Click here to read a good review on this topic).
 The actions of CDNF. Source: Sage
The actions of CDNF. Source: Sage
CDNF is being developed as a therapy for Parkinson’s by the biotech firm, Herantis.
The results of their Phase I/II study evaluating the safety and tolerability of CDNF in people with Parkinson’s were announced in 2020 (Click here to learn more about this and click here to read a SoPD post about this). Alternatively, you can watch this video:
Similar to the Bristol Phase II GDNF study (mentioned above), the drug was injected directly into the brain using an implanted canular system. One-third of the participants received monthly infusions of placebo and two-thirds of the participants received monthly infusions of either mid- or high-doses of CDNF for 6 months (Click here to read more about this trial).
The results indicate that the treatment was safe and well tolerated, but the company has decided “to pursue more patient-friendly modes of delivery that do not require the need for a surgical device“, rather than pursue a larger Phase II study (Source). Given the invasive nature of this procedure, this is probably a wise move.
And the company has made good progress in the development of this alternative version of CDNF. In January 2022, Herantis announced it intends to advance HER-096 – a small molecule synthetic form of CDNF – by filing for regulatory approval in the 4th quarter of 2022 to start a first-in-man clinical study (Source).
In January, Athira Pharma announced the initiation of dosing in their “SHAPE study” – a Phase 2 clinical trial of ATH-1017 (an enhancer of hepatocyte growth factor (HGF) & its receptor, MET) for the treatment of Parkinson’s dementia & dementia with Lewy bodies.
This is a randomized, placebo-controlled, double-blind study recruiting 75 individuals who will be assessed for 26 weeks. The study is scheduled to finished in June 2023 (Click here to read more about this study).
Finally, in 2021, New Zealand-based Living Cell Technologies announced that they have secured funding for its 3rd clinical trial of NTCELL in Parkinson’s. Previously the company had some disappointing results from their Phase II study (Click here to read an old SoPD post on this company), but they are now seeking to determine if NTCELL can pause/delay PD progression. Starting 2024, the company will transplant their encapsulated cells which release neurotrophic factors and participants will be monitored over a 3 year follow up period (Source).
|
Preclinical developments in neurotrophic research to look out for in 2022:
|
In addition to the research being conducted on GLP-1 agonists and neurotrophic factors, there are additional neuroprotective approaches being explored.
Neuroprotective approach: Additional
A number of research groups are exploring the idea of using plasma – the portion of blood that doesn’t contain cells – from young organisms to improve function in older creatures. The hypothesis is that this “young blood” may contain beneficial properties for older organisms.
Not quite Count Dracula, but there has been a lot of research exploring this idea (Click here to read a SoPD post on this topic). There have also been a number of clinical trials investigating this as a therapeutic approach, including for Parkinson’s.
In 2020, we saw the results of the Stanford Parkinson’s Disease Plasma Study (SPDP) published (Click here to read a SoPD post about those results).
 This was a Phase I open label clinical study testing whether young plasma infusions could be safely used on 15 people with Parkinson’s. The participants received 1 unit of young fresh frozen plasma twice a week for 4 weeks, and they were followed up for 4 weeks after the infusions ended.
This was a Phase I open label clinical study testing whether young plasma infusions could be safely used on 15 people with Parkinson’s. The participants received 1 unit of young fresh frozen plasma twice a week for 4 weeks, and they were followed up for 4 weeks after the infusions ended.
The results suggest that not only was the treatment safe and well tolerated, but elevated baseline measures of inflammation (such as blood levels of tumor necrosis factor‐α) were decreased 4 weeks after the infusions ended.
The study was too small and short to provide any meaningful measures of efficacy, but the investigators conducting the study suggested that the results “warrant further therapeutic investigations in PD”. In 2022, we are hoping to hear news of any further developments for this clinical program.
In addition to this study in Stanford, a clinical trial of a product derived from young blood plasma (called GRF6021) was scheduled to finish in 2020. This trial is being conducted by a biotech firm named Alkahest. At the end of 2018, the company announced that they have dosed the first participant in a Phase II clinical trial of their product GRF6021 in people with Parkinson’s and cognitive impairments (Click here for the press release). GRF6021 is a ‘plasma fraction’ (evidently made up of approximately 400 proteins derived from young blood – source).
At the end of 2018, the company announced that they have dosed the first participant in a Phase II clinical trial of their product GRF6021 in people with Parkinson’s and cognitive impairments (Click here for the press release). GRF6021 is a ‘plasma fraction’ (evidently made up of approximately 400 proteins derived from young blood – source).
The study was a randomised, double-blind, placebo-controlled Phase II study in 90 people with Parkinson’s and cognitive impairment. It was assessing the safety and tolerability of GRF6021 over a period of 7 months. The treatment (or placebo) was administered by intravenous infusion for 5 consecutive days at Week 1 and Week 13 of the study (Click here to read more about the details of this clinical study).
At the March 2021 AD/PD conference, the company announced that the treatment met its primary safety endpoint in the study and there were no serious adverse events were attributed to GRF6021. The results also showed some improvements in several secondary endpoints (such as the Montreal Cognitive Assessment and the PDQ-39 quality of life measure – Source). Hopefully the full results of the study will be published in 2022.
Enough talk of blood, let’s move on to a different kind of alternative approach.
One of the standout results for Parkinson’s research in 2021 (based on my humble opinion) was the results of a clinical trial of a drug called Blarcamesine (previously known as ANAVEX2-73). The drug is a Sigma-1 agonist (Click here to read a review on Sigma-1 biology) and it is being developed by the biotech company Anavex Life Sciences.
This agent was being tested in a Phase 2, double-blind, placebo-controlled study, evaluating the safety, tolerability, and efficacy of blarcamesine in 132 people with Parkinson’s with dementia. The participants were treated for 14 weeks and the assessments were primarily focused on cognitive measures (Click here to read more about the details of this trial).
 Source: Anavex
Source: Anavex
In June 2021, the results of the study were announced and the press release stated that “From baseline to end of trial at 14 weeks, MDS-UPDRS Total score improved by -10.98 points in the ANAVEX®2-73 high dose group and worsened by 3.53 points in the placebo group, an adjusted mean difference of -14.51 points (p = 0.034).”
 Source: Anavex
Source: Anavex
This is rather jaw dropping stuff.
A 14-week study is very short, and an improvement of 11 points is simply remarkable. It corresponds to a relative improvement of almost 20% over 14 weeks (18.9% to be exact).
And it is important to remember that is that this result was achieved while the participants were on their standard medication. That is to say, the participants were on their standard PD medication (e.g. L-dopa, etc) during the clinical assessments – so the improvement in total UPDRS score was on top of the response to standard medication (Click here to read a SoPD post on this topic).
Typically, individuals with PD decline in MDS-UPDRS scores by 3.99 to 7.45 points per year (Click here to read more about this), so a 10.98 point improvement is worth noting (remember this was a double-blinded study).
A 48 week open label extension study for the participants of this study is now being conducted (Click here to read more about that study). This extension is scheduled to finish in mid 2022, so hopefully we will learn more about this research later in the year, and also be given news on the future clinical development of blarcamesine.
A large Phase III clinical trial of the effects of Lingzhi (also known as Ganoderma or reishi in Japan) on disease progression in 288 people with recently diagnosed, untreated Parkinson’s was scheduled to finish in December 2021 (Click here to read more about this study). Limited preclinical research has been published on this extract of an oriental fungus in models of Parkinson’s (for examples, click here and here), but the results of this clinical trial could be something else to keep an eye out for in 2022.
|
Preclinical developments in neuroprotective additional approaches research to look out for in 2022:
|
Phew, long post.
If you have read this far in just one sitting, then I owe you a beer.
And now, we move on to 2022 clinical trials involving the third component of any ‘curative therapy’ for Parkinson’s:
COMPONENT #3. Some form of restorative therapy
Once the condition has been slowed/halted (component #1) and a neuroprotective/nurturing environment is in place to protect the remaining cells (component #2), a curative treatment for Parkinson’s will require replacing some of the cells that have been lost.
By the time a person is presenting the motor features characteristic of Parkinson’s, and being referred to a neurologist for diagnosis, they have already lost approximately 50% of the dopamine producing neurons in an area of the brain called the midbrain. These cells are critical for normal motor function – without them, movement becomes very inhibited, resulting in the slowness of movement and rigidity symptoms associated with the condition.
And until we have developed methods that can identify Parkinson’s long before the motor features appear (which would require only a disease halting treatment), some form of cell replacement therapy is required to introduce new cells to take up lost function.
Cell transplantation currently represents the most straight forward method of cell replacement therapy.
Cell Transplantation
Traditionally, the cell transplantation procedure for Parkinson’s has involved multiple injections of developing dopamine neurons being made into an area of the brain called the putamen (which is where much of the dopamine naturally produced in the brain is actually released). These multiple sites allow for the transplanted cells to produce dopamine in the entire extent of the putamen. And ideally, the cells should remain localised to the putamen, so that they are not producing dopamine in areas of the brain where it is not desired (possibly leading to side effects).

Targeting transplants into the putamen. Source: Intechopen
Postmortem analysis – of the brains of individuals who have previously received transplants of dopamine neurons and then subsequently died from natural causes – has revealed that the transplanted cells can survive the surgical procedure and integrate into the host brain. In the image below, you can see rich brown areas of the putamen in panel A. These brown areas are the dopamine producing cells (stained in brown). A magnified image of individual dopamine producing neurons can be seen in panel B:

Transplanted dopamine neurons. Source: Sciencedirect
The transplanted cells take several years to develop into mature neurons after the transplantation surgery. This means that the actually benefits of the transplantation technique will not be apparent for some time (2-3 years on average). Once mature, however, it has also been demonstrated (using brain imaging techniques) that these transplanted cells can produce dopamine.
As you can see in the images below (a bird’s eye view of the brain from above), there is less dopamine being processed (indicated in red) in the putamen on each side of the Parkinsonian brain on the left than the brain on the right (several years after bi-lateral – both sides of the brain – transplants):

Brain imaging of dopamine processing before and after transplantation. Source: NIH
The old fashioned approach to cell transplantation involved dissecting out the region of the developing dopamine neurons from multiple donor embryos (these are referred to as fetal transplants – different to the fecal transplants discussed further above). The tissue was broken into small pieces that could be passed through a tiny syringe, and this was injected into the brain of a person with Parkinson’s.
The people receiving this sort of transplant would require ‘immunosuppression treatment’ for long periods of time after the surgery. This additional treatment involves taking drugs that suppress the immune system’s ability to defend the body from foreign agents. This step is necessary, however, in order to stop the body’s immune system from attacking the transplanted cells (which would not be considered ‘self’ by the immune system), allowing those cells to have time to mature, integrate into the brain and produce dopamine.
|
SMALL SIDE NOTE HERE: It is very important for all readers of this post to appreciate that cell transplantation for Parkinson’s is still extremely experimental. Anyone declaring otherwise (or trying selling a procedure based on this approach) should not be trusted. There are no cell therapy approaches for Parkinson’s that have been approved by any medical regulators. While I appreciate the desperate desire of the Parkinson’s community to treat this condition ‘by any means possible’, bad or poor outcomes for this technology could have serious consequences for the individuals receiving the procedure and negative ramifications for all future research in the stem cell transplantation area. |
There are a number of ongoing clinical trial for cell transplantation in Parkinson’s. Firstly, there is the TRANSEURO study which is using the traditional fetal cell-based approach to develop new protocols for novel methods of cell replacement therapy.
This trial is an open label study, involving 13 subjects (Click here to read more about this). This study finished in 2021 and we will be learning the results of this project in 2022.
There are also a number of trials evaluating approaches involving the expansion of stem cells in cell culture, partially maturing the cells towards a dopamine neuron fate before transplantation. The first of these is a Phase 1 clinical trial being conducted in Kyoto, Japan.
The ongoing Kyoto cell transplantation trial is being conducted by researchers at the Center for iPS Cell Research and Application (or CiRA).
 In 2017, the researchers behind this trial published a report evaluating their IPS cells in primate models of Parkinson’s (Click here to read more about that research report).
In 2017, the researchers behind this trial published a report evaluating their IPS cells in primate models of Parkinson’s (Click here to read more about that research report).
Information provided by Kyoto University (Click here for the study website) and the Japanese media has indicated that this Phase I/II clinical trial aims to investigate the safety and efficacy of transplanting human IPS cell-derived dopaminergic progenitors into the brains of people with Parkinson’s (what stage of PD has not been disclosed). The study is very small, involving just 7 participants – who are all Japanese individuals with Parkinson’s. The participants in the study will be followed and assessed for 2 years post transplantation
According to the CIRA 2018 annual report, the researchers began enrolling patients on 1st August and the first patient was transplanted in October (2018 – Click here to read the annual review). The investigators stated that the gentleman who was transplanted will be monitored for 2 years.
The team leading the Kyoto IPS-cell derived transplantation trial for Parkinson’s provided an update late last year. The team said that none of the three transplanted patients who have been transplanted developed any side effects (>12 months post surgery). In addition, they were planning 4 more transplants going ahead (I’m not sure how much COVID-19 may have slowed that progress down).
The Kyoto researchers are also working very closely with the pharmaceutical company Sumitomo Dainippon Pharma, which announced in March of this year that they were planning to begin US based clinical trials in 2022 of the iPS-derived cell approach for cell transplantation in Parkinson’s (Click here to read more about this).
Sumitomo has been working on better manufacturing and selection techniques for iPS cell-derived dopaminergic progenitor cells (Click here to read more about this).
The second stem cell-based cell transplantation clinical trial was initiated in 2018 by a research group from China led by Professor Qi Zhou, a stem-cell specialist at the Chinese Academy of Sciences Institute of Zoology.
This study is taking place at The First Affiliated Hospital of Zhengzhou University in Henan province, and involves 10 participants being injected with neuronal-precursor cells derived from embryonic stem cells (Click here to read more about this trial). The study was scheduled to finish in late 2020, but we are yet to hear any news regarding this study (Click here to read a previous SoPD post on this topic).
A third trial is being conducted by Allife Medical Science and Technology Co. in China.
 This study of 10 participants is also a safety trial, it is also using neuronal-precursor cells derived from embryonic stem cells, and it was scheduled to finish in 2021 (Click here to read more about this study), so we will hopefully learn the outcome of this study in 2022.
This study of 10 participants is also a safety trial, it is also using neuronal-precursor cells derived from embryonic stem cells, and it was scheduled to finish in 2021 (Click here to read more about this study), so we will hopefully learn the outcome of this study in 2022.
There has also been a clinical study conducted by International Stem Cell Corp (ISCO) in Australia. The company recently announced the completion of that study and that the treatment was safe (Click here to read more about this). Here at the SoPD we have been very concerned about this trial (Click here and here to read previous posts about this), so it was reassuring to learn of this outcome.
One biotech company focused on cell transplantation for Parkinson’s that we have a lot more confidence in is Aspen Neuroscience.
 This company has been developing two products: ANPD001 – an autologous neuron replacement therapy in Parkinson’s, and a second product ANPD002 which is “a gene-edited autologous neuron replacement therapy for familial forms of Parkinson’s” which appears to be initially focused on GBA-associated Parkinson’s (Source).
This company has been developing two products: ANPD001 – an autologous neuron replacement therapy in Parkinson’s, and a second product ANPD002 which is “a gene-edited autologous neuron replacement therapy for familial forms of Parkinson’s” which appears to be initially focused on GBA-associated Parkinson’s (Source).
 In April 2020, Aspen announced that they had raised US$70 million in Series A funding to help progress development of their program (Click here to read a SoPD post on this topic). They are keen to start clinical testing in the very near future.
In April 2020, Aspen announced that they had raised US$70 million in Series A funding to help progress development of their program (Click here to read a SoPD post on this topic). They are keen to start clinical testing in the very near future.
Another biotech firm that is seeking to start clinical testing their cell transplantation product is Fujifilm/Cellular Dynamics.
They have a very active cell transplantation program under preclinical development for Parkinson’s (Source), and we have recently learned that a clinical trial is planned to start soon in a collaboration with ASU-Banner Neurodegenerative Disease Research Center at Arizona State University and the Van Andel Institute (Michigan).
The planned trial is made particularly interesting as the investigators will be seeking individuals with Parkinson’s who also carry a variant in their PARKIN gene (Source). PARKIN-associated PD is typically associated with the loss of dopamine neurons, but not Lewy body pathology, and they generally do not have some of the other features of PD (loss of smell, etc). These motor-specific characteristics make these individuals uniquely appropriate for testing whether cell transplantation is a viable treatment for restoring motor function (Click here to read a previous SoPD post on this topic).
Fujifilm/Cellular Dynamics has been working on cell transplantation in Parkinson’s for a long time and it is encouraging to see that their efforts are now moving to the clinic.
 Source: Fujifilm
Source: Fujifilm
And finally, in a fascinating presentation at the World Parkinson’s Coalition’s virtual meeting in May 2021, Dr Agnete Kirkeby provided some of the details regarding the STEM-PD clinical trial that will be starting in 2022.
This will be a collaboration between the University of Lund, Cambridge University and the pharmaceutical company Novo Nordisk (Source).
 The details provided during the presentation indicate that the STEM-PD trial will be small (just 8 patients) with a primary endpoint of safety (determined at 12 months post transplant) and a secondary endpoint of efficacy (measured at 3 years post transplant). The participants will have been diagnosed more than 5 years ago, with mild/moderate symptoms, and they will receive one of two doses.
The details provided during the presentation indicate that the STEM-PD trial will be small (just 8 patients) with a primary endpoint of safety (determined at 12 months post transplant) and a secondary endpoint of efficacy (measured at 3 years post transplant). The participants will have been diagnosed more than 5 years ago, with mild/moderate symptoms, and they will receive one of two doses.
Some of these details may change before the trial officially starts, but it is very encouraging to see industry and academic collaboration in this endeavour.
|
# # # # New developments in the field of cell transplantation research to look out for in 2022: In addition to the active (and near) clinical programs, there are a number of preclinical efforts underway that we will be watching out for any news this year:
There are also new biotech companies popping up that are exploring the concept of directly converting cells in the brain into dopamine neurons as a therapy for Parkinson’s. These include NeuExcell Therapeutics who have an active research program for Parkinson’s (source), as well as other biotech companies like Cambridge (UK)-based Mogrify. # # # # |
Ok, that’s it.
I think we are done.
It’s not everything, but it will certainly give you a flavour of where things currently stand and what to look out for in 2022, regarding clinical trials for disease modification in Parkinson’s.
I would be remise if I did not make one last statement regarding the need to check expectations.
While the mammoth list of clinical trials above bodes well for the future of Parkinson’s treatments, there are no guarantees that any of them are actually going to work. In addition, we must remember that a clinical trial is supposed to be an unbiased evaluation of a treatment. If we enter a clinical trial with any expectation (as a participant or observer), we are – by definition – biased. We should approach all of these trials with no expectations.
I should also note that one of the limitations of the ‘shopping list’ of trials above is that there was no mention of research into exercise or diet, and they certainly deserve to be mentioned. I have focused on small pharmacological and new biologics approaches simply because they are easier to assess in the proposed structure. Exercise and diet probably deserve their own posts, but I am not sure where to even begin – anyone with some thoughts on this would be welcome to contact me or leave a comment below.
SUMMARY: The main takeaways
The amount of research being conducted is a very positive sign, and it suggests that 2022 will be an extremely productive year for Parkinson’s research. The breadth of approaches now being applied to Parkinson’s is also very encouraging, and in the next few years we may have answers to some fundamental questions regarding the biology that may be underlying many cases of PD (I am thinking of alpha synuclein and LRRK2 in particular).
There is certainly going to be a lot of clinical trial results being announced in 2022! The top 5 clinical trial results that I will be looking out for this year are:
- The Phase II “UP study” results – evaluating UDCA in Parkinson’s
- The Phase II Lixisenatide study results – more data on GLP-1R agonists in PD
- The Phase II Liraglutide study results – even more data on GLP-1R agonists in PD
- The Phase II Peptron study results – lots of data on GLP-1R agonists in 2022
- The Phase II Deferiprone study results – addressing an important question
Here at SoPD HQ, we will also be watching out for lots of merger and acquisitions activity in the biotech world this coming year. Big Pharmaceutical companies have large war chests totaling over $500 billion to spend in 2022 (Source), and a large number of patents expiring on many blockbuster products. They will be looking to replace those revenue streams and there are certainly a lot of interesting approaches for Parkinson’s deep into clinical development now. Over the last few years, there has been a very positive trend of big pharma companies investing in intellectual property focused on Parkinson’s. For example:
- Biogen tied a knot with Denali (Click here to read an SoPD post about this).
- Sanofi signing a licensing deal with the South Korean biotech firm ABL Bio to develop and commercialise an anti-alpha synuclein immunotherapy (Source).
- Bayer acquired Asklepios BioPharmaceutical which then merged with Brain Neurotherapy Bio -the GDNF gene therapy biotech firm (Click here to read a SoPD post about this).
- Bayer also purchased BlueRock Therapeutics – the stem cell transplantation company last year – click here to read a SoPD post about that).
- Bial purchased Lysosomal Therapeutics (Click here to read more about this)
- Eli Lilly acquired GBA-associated Parkinson’s gene therapy company Prevail Therapeutics (Click here to read a SoPD post on this).
- Novo Nordisk getting involved in cell transplantation for Parkinson’s with academic collaborators (Source).
- Likewise, Sumitomo Dainippon Pharma getting involved in cell transplantation for Parkinson’s with academic collaborators (Source).
- AC Immune acquires assets of AFFiRiS (Click here to read a SoPD post about this).
- Inflammasome-focused biotech firm Inflazome was bought by Roche (Click here to read more about this).
- AbbVie entering into a purchase right agreement with Mitokinin (Click here to read more about this).
- And AbbVie establishing a collaboration with Caraway Therapeutics to focus on development of TMEM175 modulators in Parkinson’s (Source).
And with bulging pockets, we can expect more acquisitions and partnering deals to come in 2022. Any fears that big pharma companies are quitting neurodegeneration appear to be misplaced – with aging demographics in the Western world, the market opportunities are simply too big for these enormous companies to ignore.
All of this collective activity provides encouraging signs for future research focused on finding new therapies for slow, stopping and reversing Parkinson’s.
But now it’s time to give the fingers (and brain) a wee rest.
 All of the material on this website is licensed under a
All of the material on this website is licensed under a
Creative Commons Attribution 4.0 International License
You can do whatever you like with it!
EDITOR’S NOTE: The information provided by the SoPD website is for information and educational purposes only. Under no circumstances should it ever be considered medical or actionable advice. It is provided by research scientists, not medical practitioners. Any actions taken – based on what has been read on the website – are the sole responsibility of the reader. Any actions being contemplated by readers should firstly be discussed with a qualified healthcare professional who is aware of your medical history. While some of the information discussed in this post may cause concern, please speak with your medical physician before attempting any change in an existing treatment regime.
The author of this post is an employee of Cure Parkinson’s, so he might be a little bit biased in his views on trials supported by the trust. That said, the trust has not requested the production of this post, and the author is sharing it simply because it may be of interest to the Parkinson’s community.
In addition, many of the companies mentioned in this post are publicly traded companies. That said, the material presented on this page should under no circumstances be considered financial advice. Any actions taken by the reader based on reading this material is the sole responsibility of the reader. None of the companies have requested that this material be produced, nor has the author had any contact with any of the companies or associated parties. This post has been produced for educational purposes only.
The banner for today’s post was sourced from Weknowyourdreams
 All of the material on this website is licensed under a
All of the material on this website is licensed under a
Creative Commons Attribution 4.0 International License
You can do whatever you like with it!
The banner for today’s post was sourced from Weknowyourdreams



























































